P's exam 3 study guide
From Iusmphysiology
- started here on 03/22/11
[edit] Kidney functions
[edit] The National Kidney Disease Education Program
- 300K+ people have end stage kidney disease.
- It is expensive to take care of end stage kidney disease: 28 billion in 2010 (8% of the Medicare/caid budgets).
- 11% of the US population has chronic kidney disease.
- Testing and therapy for chronic kidney disease are inadequately applied.
- Symptoms don't result until renal failure is well progressed.
- Get regular checkups.
[edit] Homeostasis
- Claude Bernard suggested the idea of homeostasis: "constancy of the internal milieu is the essential condition to a free life".
- Walter Canon developed the idea of homeostasis and gave it said name.
- Homer Smith explains why the kidney filters everything and then has to reabsorb most of it.
- "Recognizing that we have the kind of blood we have because we have the kind of kidneys we have, we must acknowledge that our kidneys constitute the major foundation of our physiological freedom."
- Recall that mammal (and all land animals) came from sea animals.
- The kidneys have evolved from an environment where there was too much water (sea) to an environment where there is too little water (land).
- And many diverse animals keep their ions (Na, K, Ca, Mg, and Cl) at very consistent proportions.
- And close to the concentrations of sea water.
[edit] Drinking urine is good for you
- Drinking urine is NOT good for you!
[edit] Kidney functions
- The kidneys have many functions, all of which focus on homeostasis of the fluid.
- Most important the amount of Na.
- Results in regulation of the amount of water retained.
- The kidneys regulate the osmotic pressure of the body fluids by retaining or losing water.
- The kidneys regulate concentrations of 8 major ions / molecules: Na, K, Mg, Ca, Cl, HCO3- (and therefore H+), phosphate (PO3-) and sulfate (SO4-).
- The kidneys eliminate waste products and foreign compounds (think drugs and urea).
- The kidneys regulate extraceullar fluid volume.
- The kidneys regulate arterial blood pressure.
- The kidneys have specialized metabolic functions:
- Gluconeogenesis
- Degradation of polypeptide hormones
- Synthesis of ammonia from aas.
- The kidneys add important substances to the blood:
- Erythropoietin
- 1,25OH VitD (calcitriol)
- Prostaglandins and thromboxane
- Renin
- Kallikrein
[edit] Erythropoietin
- Erythropoietin (EPO) is released by cells of the renal cortex in response to hypoxia.
- EPO (erythropoietin) acts on the bone marrow to increase RBC proliferation, matruation and release.
- As more RBCs are made, oxygen carrying capacity goes up.
- In chronic renal disease, too little EPO is generated by the kidney and anemia results.
- In blood doping, EPO was used to increase oxygen carrying capacity until we became able to determine the difference between endogenous and exogenous EPO.
- Treatment of anephritic patients with EPO is far more effective than the cycles of transfusion that would otherwise be required.
[edit] Vitamin D
- Vitamin D is hydroxylated once at the liver and once at the kidney.
- Recall that 7-dehydrocholesterol is obtained from the diet, converted to vitamin D3 at the skin, hydroxylated at the 25 position at the liver, and then at the 1 position in the kidney.
- The kidney adds the second hydroxyl group at the 1 position.
- Calcitriol (1,25OH vit D) is the biologically active form of vitamin D.
- Cacitriol causes increased Ca absorption at the gut and increased Ca mobilization at the bone (by potentitiating PTH's action, from the parathyroid).
- In renal disease, vitamin D is not hydroxylated well and so the body has too little biologically active vitamin D (calcitriol).
- As the kidney fails, increased phosphate in the blood (hyperphosphatemia) and decreased renal tubular mass lead to decreased ability of the kidney to hydroxylate 25OH VitD to calcitriol (1,25 vit D3).
- Phosphate levels go up because phosphate cannot be lost in the filtrate.
- Also, high phosphate can crystalize and cause kidney problems.
- Decreased calcitriol generation has a series of effects (literally, a series):
- This results in poor calcium absorption at the gut.
- Because there is poor Ca absorption at the gut, there is also hyperparathyroidism as the parathyroid works overtime to signal to the gut and bone to keep the Ca levels high despite poor absorption.
- And because the parathyroid is calling on the bone to release lots of Ca to keep serum levels normal, the bone becomes demineralized (bone disease).
- Finally, there is vascular calcification.
Why is there calcium calcification? "Patients with chronic kidney disease are at risk for vascular calcification because of multiple risk factors that induce vascular smooth muscle cells to change into a chondrocyte or osteoblast-like cell; high total body burden of calcium and phosphorus due to abnormal bone metabolism; low levels of circulating and locally produced inhibitors; impaired renal excretion; and current therapies." per JASN
[edit] Prostaglandins and Thromboxanes
- Prostaglandins and thromboxanes are made from phospholipds via cyclo-oxygenase enzymes like COX1 and COX2.
- Cyclo-oxygenase enzymes (COX1 and COX2) are constitutively activated in the kidney.
- The kidneys make lots of prostaglandins and thromboxanes which have effects on the kidney, itself.
- Prostaglandins:
- Prostaglandins increase renal blood flow.
- Prostaglandins increase Na excretion (increases water reabsorption, increases blood pressure).
- Prostaglandins increase renin release (increases water reabsorption, increases blood pressure).
- Recall that renin converts angiotensinogen to angiotensin 1 which gets converted to antiotensin 2 (by ACE in the lung) which causes blood vessels to constrict (elevates blood pressure) and causes release of aldosterone (from the glomerulosa zone of the adrenal cortex) which causes the kidney to reabsorb more Na and therefore more water (elevates blood pressure).
- Prostaglandins inhibit the actions of ADH on the kidney (decreases water absorption, decreases blood pressure).
- Recall that ADH (anti-diuretic hormone, AVP arginine vasopressin, from the posterior pituitary) causes the kidney to reabsorb water.
- Thromboxanes are vasoconstrictors (increases blood pressure).
- PGE2 and PGI2 are the main prostaglandins.
[edit] Renin-angiotensin system
- The overall scheme is that angiotensin is present in the blood, renin cuts it into angiotensin 1, ACE cuts angiotensin 1 into angiotensin 2.
- Note that angiotensinogen comes from the liver, renin comes from the kidney, and ACE (angiotensin converting enzyme) comes from the lungs.
Did Homor Smith explain why it makes sense that one enzyme should come from the lungs (ACE) and the other from the kidneys (renin)? No...
- Angiotensin 2 is all about increasing blood pressure and therefore has effects on the vasculature (think constriction), the adrenal cortex (think aldosterone, and the brain (think thirst).
- Angiotensin 2 causes vasculature to constrict, thus increasing the blood pressure.
- Angiotensin 2 causes the adrenal cortex (the zona glomerulosa) to release aldosterone which causes the kidney to increase Na reabsorption (and therefore water reabsoprtion), thus increasing the blood pressure.
- Angiotensin 2 causes the brain (posterior pituitary) to release AVP (arginine vasopressin = ADH) which causes the kidney to put more aquaporin proteins on the renal tubule epithelial cells which increases water reabsorption, thus increasing the blood pressure.
- ACE inhibitors inhibit the conversion of angiotensin 1 to angiotensin 2 and thus help keep blood pressure low (by decreasing water reabsorption (reduced aldosterone and AVP) mostly and also by decreasing vascular constriction).
[edit] Renal kallikrein enzyme system
- The renal-kallikrein system serves to dilate the vasculature (so it has mostly an opposite affect as the renin-angiotensin system, in terms of the vasculature).
- The general pathway is kininogen to kinins via kallikrein, then kinins to inactivated peptides via kininases.
- Kininogens are made primarily by the liver, but also by some other tissues.
- Kallikrein is made by the kidney.
- The active molecules are the kinins, like bradykinin.
- Bradykinin increases production of NO and prostaglandins that act on the smooth muscle cells of the vasculature and therefore kinins are potent vasodilators.
- Recall that the kidney makes lots of prostaglandins through the cyclo-oxygenase genes.
- ACE (recall that it converts angiotensin 1 to angiotensin 2 which goes on to increase blood pressure in many ways) is a kininase so it helps to increase blood pressure by getting rid of the kinins (like bradykinin) that are floating around trying to decrease the blood pressure.
[edit] The nephron
- The nephron is the basic structural and functional unit of the kidney.
- Each kidney is supplied by a renal artery, a renal vein, and a ureter which enter at the renal hilus.
- The outside of the kidney is the renal capsule.
- Deep to the capsule is the cortex.
- Within the cortex framework are medullary pyramids in which are the medulla.
- The beginning of the drainage structures are the minor calyces, then the major calyces, then the renal pelvis, and finally the ureter.
- The area of the renal pelvis and the calyces combined is the renal sinus.
[edit] Blood vessels of the kidney
- Recall that there is the cortex and medulla of the kidney.
- The cortex and medulla are histologically distinct.
- Within the medulla, we define two distinct areas: the outer medulla and the inner medulla.
- The kidney blood supply is specialized to allow the kidney to perform it's filtering duties.
- Each nephron has its own blood artery and vein.
- The overall flow is renal artery -> arcuate artery -> cortical radial artery -> afferent arteriole (one for each glomerulus) -> glomerulus (either a superficial cortical glomerulus or a juxtamedullary glomerulus) -> efferent arteriole -> peritubular arteries -> arcuate vein -> renal vein.
- The peritubular capillaries deliver oxygen to the cells of the tubule and serve to reabsorbed material from the interstitial fluid back into the blood.
- The superficial cortical glomerulus and the juxtamedullary glomerulus differ in their function and in their location within the kidney.
- The descending vasa recta provide another route for blood.
- The descending vasa recta branch off the efferent arterioles (that is, distal to the glomerulus) of the juxtamedullary glomeruli.
- The descending and ascending vasa recta lie parallel to an individual tubule.
- Note that the bowman's capsule is the set of epithelial cells that surround the gomerular capillaries.
- The functional unit of the kidney is the nephron, of which there are about 1 million in each kidney.
- 85% of the nephrons proceed from the superficial cortex to the outer medulla and have superficial cortical glomeruli.
- 15% of the nephrons proceed from the deep cortex to the inner medulla and have juxtamedullary glomeruli.
- The tubule (which has descending thick, descending thin, and ascending thin, ascending thick segments) leads to the collecting duct.
[edit] Renal osmolarity gradient
- The through filtration and blood flow, the kidney maintains an osmotic gradient along the cortex-medulla axis.
- There are lots of capillaries surrounding the nephrons.
[edit] Blood flow to the kidneys is high
- 25% of the cardiac output goes to the kidneys.
- Flow by gram of tissue is higher than the brain!
- High blood flow is important for driving a high filtration rate.
- The 3 liters of plasma is filtered every 24 minutes (which is 50-60 times per day).
[edit] Major processes involved in urine formation
- The blood is "filtered" at the glomerulus; small particles flow out of the blood into the "filtrate".
- Some material from the filtrate is "reabsorbed" in the kidney tubule; epithelial cells reabsorb ions and such back into the interstitial fluid / blood.
- Some materials are "secreted" into the filtrate; epithelial cells can put metabolites and such into the filtrate (urine).
[edit] Proximal tubule
- 2/3 of the filtrate is reabsorbed in the proximal tubule.
- The proximal tubule is the epithelial tract that is nearest the glomerulus.
- The proximal tubule is a specialized tissue for reabsorption and secretion:
- Neighboring epithelial cells have tight junctions to keep "stuff" from passing without an active transport mechanism (either reabsorption or secretion).
- The epithelial cells have microvilli (a brush border) to increase the surface area and the ability to reabsorb / secrete.
- The epithelial cells have many microvilli to produce lots of ATP for all the active transport that must occur.
- Also, lots of Na / K ATPase activity to maintain a Na gradient to run all the active transport.
- The epithelial cells sit on a basement membrane to provide structure and order to the single layer of cells.
[edit] The juxtaglomerular apparatus (JGA)
- The juxtaglomerular apparatus is important for two major functions: the release of renin and feedback on the tubulo-glomerular feedback.
- Recall that the release of renin will convert angiotensinogen to AT1 (angiotensin 1) which will get converted to angiotensin 2 (at the lungs by ACE) which will cause blood vessels to constrict and aldosterone to be released, thus increasing blood pressure.
- The juxtaglomerular apparatus is called an apparatus because it functions by the coordinated activity of three cell types from two separate structures of one nephron.
- Macula densa cells are epithelial cells found on the thick ascending tubule of the nephron.
- The ascending tubule is coming up (toward cortex) from the loop of Henle.
- Macula densa cells seem to be more columnar in shape than their more cuboidal, normal endothelial cells.
- Extraglomerular mesangial cells are also found on the thick ascending tubule.
- Extraglomerular mesangial cells hold the JGA structure together.
- Extraglomerular mesangial cells may also convert stuff released by the macula densa.
- Granular cells (also called juxtaglomerular cells) are endothelial cells found in the wall of the afferent arteriole.
- Macula densa cells are epithelial cells found on the thick ascending tubule of the nephron.
[edit] Tubulo-glomerular feedback
- Tubulo-glomerular feedback describes how the concentration of NaCl in the tubule (that is, in the filtrate) can be used to affect the GFR (glomerular flow rate) by changing the diameter of the afferent arterioles that lead to the glomerulus.
- Note that this it taking place within a single nephron; as the tubule ascends back toward the cortex, it passes by it's own glomerulus and therefore can have a very rapid affect on the GFR.
- The macula densa cells detect the NaCl concentration in the tubule and send molecular signals to the afferent arterioles.
- Sensing of the NaCl concentration is a model of filtrate flow.
- Why detect the NaCl?
- Recall that the tubule is all about reabsorbing important ions and molecules from the filtrate, including Na and Cl (the body doesn't want to lose them).
- So if NaCl concentration is high at the distal tubule (that is, near the macula densa), then we know the filtrate needs to move more slowly in order to reabsorb more of the Na and Cl.
- If NaCl concentration is low at the distal tubule, then we know the filtrate can be moved along more quickly.
- Now recall that the flow rate of the filtrate is a function of glomerular filtration rate which is a function of the blood pressure difference between the afferent and efferent arterioles of the glomerulus.
- So, we should change the blood pressure of the afferent arteriole if we want to change the flow rate of the filtrate (and therefore affect the reabsorption levels).
- The macula densa cells can respond to both high and low NaCl concentrations:
- When NaCl concentrations are low, filtrate flow rate is too slow. The macula densa cells signal to the granular cells to release renin.
- Recall that renin will increase blood pressure systmeically because angiotensin 2 causes vasoconstriction, aldosterone release at the adrenal gland, and AVP release at the posterior pituitary.
- It makes sense that low flow rate should lead to release of renin by the granular cells because increased blood pressure (at the afferent arteriole) causes an increase in filtrate formation (increased blood flow will push more fluid through the fenestrations at the glomerulus and thus generate more filtrate).
- Note that this will not cause immediate vasoconstriction, because the renin-angiotensin pathway must run its course, which includes a trip through the lungs (think ACE).
- Note that macula densa cells signal to granular cells via PGE2, a prostaglandin produced by COX2 in the macula densa cells.
- The granular cells have the EP4 receptor for PGE2 from the macula densa cells.
- The granular cells of the afferent arteriole are well named because they have granules full of renin.
- When NaCl concentration levels are high, filtrate flow is too fast. The macula densa cells signal to the endothelial cells of the afferent arteriole to vasoconstrict.
- ATP is released by the macula densa cells and is converted to adenosine (a vasoconstrictor) to decrease blood flow of the afferent arteriole and thus decrease filtrate production.
- Adenosine binds to the A1 receptors of the endothelial cells of the afferent arteriole to cause vasoconstriction.
- When NaCl concentrations are low, filtrate flow rate is too slow. The macula densa cells signal to the granular cells to release renin.
[edit] Angiotensin 2
- Recall that angiotensin 2 causes systemic vasoconstriction, release of aldosterone at the adrenal cortex (glomerulosa), and release of ADH (AVP) at the posterior pituitary.
- At low concentrations, angiotensin 2 causes efferent arteriole constriction.
- This causes a decrease in renal blood flow (RBF).
- This will cause an increase in the difference between the afferent arteriole blood pressure and the efferent arteriole blood pressure, and thus and increase in GFR.
- At high concentrations (as in trauma and emergencies), both the afferent and efferent arterioles vasoconstrict.
- This causes a decrease in RBF (renal blood flow).
- This causes a decrease in GFR (glomerular filtration rate).
- This makes sense because you want to conserve all the fluid you can in an emergency.
- Angiotensin 2 levels are severely elevated in emergency situations because sympathetic nerves directly stimulate the granular cells of the juxtamedullary apparatus to release their renin and thus much more angiotensin 2 is generated than when macula densa cells signal for renin release.
[edit] Micturition = Urination
- Micturition is a voluntary control in adults.
- Children do not obtain voluntary control of micturition until 2-3 years of age.
- The tension in the wall of the bladder adjusts as the bladder fills to keep pressure from building up.
- One generally feels the need to micturate at about 150-250 ml and loses control of voluntary micturition at 700 ml.
- The ureters have a smooth muscle layer that forces urine along the tract.
- This is important for outer space travel and to counteract the counter pressure of the fluid already in the bladder.
[edit] Renal plasma clearance
- There are multiple forces that cause a net movement of plasma out of the blood at the glomerulus into the filtrate:
- PiGC is the glomerular capillary colloid osmotic pressure; that is, the colloid pressure of the blood (forces into the blood)
- PGC = is the glomerular capillary hydrostatic pressure; that is, the hydrostatic pressure from blood flow (forces into filtrate)
- PBS = Bowman space hydrostatic pressure; that is, the hydrostatic pressure of the filtrate (forces into the blood)
- We don't include the colloid pressure of the Bowman space because there it is negligible (which makes sense because proteins don't get filtered)
- One measure of renal function is the renal plasma clearance, defined as ml plasma / min that are cleared of a substance.
- The equation is C * P = U * V where
- C = clearance of the substance (the unknown, the indicator of kidney function)
- P = plasma concentration of the substance
- U = urine concentration of the substance
- V = urine flow rate
- C = UV/P
- As an example: the plasma concentration is 2 mg/ml, the urine concentration is 6 mg/min.
- C = UV/P = 6 mg/min / 2 mg/ml = 3 min/ml
- Note that v-dot (a "v" with a dot over it) is commonly used to denote a rate of flow; the dot distinguishes it from a volume.
- Inulin is a good test compound because it is biologically inert and the kidney reabsorbs nearly zero of the filtered inulin.
- Because very little inulin is reabsorbed, the amount of filtered inulin is equal to the amount of excreted inulin.
- Therefore, the GFR = C = UV/P.
- One might wonder why the urinary inulin is more concentrated than the plasma inulin.
- Its because while none of the inulin gets reabsorbed, so many of the other filtered molecules (including water) do get reabsorbed.
- stopped here on 03/22/11
- started here on 03/23/11
[edit] Endogenous creatinine clearance
- Creatinine is a useful endogenous molecule for measuring kidney function because there is little to no tubular reabsorption of creatinine.
- We can't use inulin with humans.
- In fact, creatinine is easily filtered and even actively secreted in the proximal tubule.
- Therefore, when plasma creatinine levels rise it is usually the result of poor renal function and a decreased GFR.
- When plasma creatinine levels double, GFR has decreased by 1/4 (25%).
[edit] MDRD equation for estimating GFR
- This is a well studied equation.
- Age, gender, and AA race are taken into account.
- As GFR declines, creatinine goes up.
[edit] Example GFR data
- A normal GFR is about 125 ml plasma / min.
- A normal day has about 1440 minutes in it.
- So a normal kidney filters 125 ml plasma / min * 1440 min = 180 L / day.
- And we have about 3.5 L of plasma (filtered 50 times per day) and 14 L of extracellular fluid (filtered 13 times per day).
[edit] Filtration fraction
- Another useful metric for renal function is the ratio (fraction) of fluid filtered to renal plasma flow (filtration fraction = GFR / 55% of RPF).
- This fraction will tell us how much of the plasma is filtered with each pass of a unit of blood through the kidney.
- Recall that a normal GFR = 125 ml plasma / min.
- Recall that a normal RPF = 660 ml / min.
- Note that a normal RBF (renal blood flow) is 1200 ml blood / min and that 55% of the volume of blood is plasma so 0.55 * 1200 = 660 ml of plasma.
- So a normal fraction is 125 / 660 = 0.19.
- So around 20% of the plasma is filtered with each pass through the kidney.
[edit] PAH clearance
- The clearance of p-aminohippurate (PAH) has been shown to model well the renal plasma flow (RPF).
- Note that PAH is not normally found in blood plasma.
- Also, PAH is cleared in a single pass through the kidney because it is filtered and highly secreted.
- More than 90% of the PAH is cleared in one cycle of the blood through the kidneys.
- An extraction ratio is the amount of a compound entering the kidney versus the amount excreted in the urine.
- PAH has an extraction ratio of nearly 1, meaning that nearly all of the PAH that enters the kidney gets filtered out into the filtrate urine.
- Therefore, the clearance rate of PAH is a good estimate of the renal plasma flow (note that this is not the same as renal blood flow, this is how much plasma is flowing through the kidney).
[edit] Renal blood flow versus renal plasma flow
- Renal blood flow and renal plasma flow are not the same value!
- RBF = RPF / (1 - hematocrit)
- Recall that a normal renal plasma flow (RPF) is 660 ml plasma / min.
- Recall that a normal plasma / hematocrit ratio is 0.45.
- So a normal RBF (renal blood flow) is 660 ml plasma / min / (0.55 ml plasma / ml blood) = 1200 ml blood / min.
[edit] PAH and renal blood flow
- We can calculate the renal blood flow using PAH administration.
- Give PAH until the plasma level is steady.
- Collect a timed urine sample and a blood sample.
- Measure the concentration of PAH in the plasma and the urine.
- Calculate the clearance rate of PAH (C).
- Recall that C = UV/P.
- Remember that V = urine flow rate, not the volume!
- This is the effective renal plasma flow (eRPF).
- Recall that C = UV/P.
- Measure the blood hematocrit (Hct)
- Then calculate the renal blood flow (RBF).
- RBF = RPF / (1 - Hct)
- continued on to Renal blood flow, glomerular filtration on 03/23/11.
- continued here from Kidney functions on 03/23/11
[edit] Renal blood flow and glomerular filtration
[edit] Data for a resting, young adult, 70 kg man
- A healthy man's blood flow distribution is like this:
- 1200 ml / min to the liver
- 1200 ml / min to the kidneys
- 750 ml / min to the brain
- 250 ml / min to the heart
- Note that the kidneys receive 25% of the cardiac output and the highest proportion of blood flow by weight.
- Also note that the kidneys use 20 ml of Oxygen / min.
- This is mostly to drive ATP production for active Na reabsorption.
- The kidneys have low oxygen extraction from their blood supply.
[edit] Blood flow rate of the kidney
- The blood flow rate can be described by the number of ml of blood that flow in a certain time (min) to a certain mass of tissue (g).
- ml / min / g
- The flow rate within the kidney is different depending on the location.
- In the cortex, blood flow rate is high because a high flow rate encourages filtration which is the job of the cortical glomeruli.
- In the medulla, the blood flow rate is lower because a low rate will not sweep away all the molecules of the interstitial fluid that are setting up the osmotic gradient that pulls nutrients out of the filtrate.
- Note that this low blood flow rate is still high enough to provide life-sustaining nutrients to the cells within the medulla.
[edit] Autoregulation of renal blood flow and GFR
- The kidney is engineered to have autoregulation of RBF and GFR.
- This autoregulation keeps small, normal changes in arterial blood pressure from changing the GFR.
- This is important because Na and H20 loss are a function of GFR (recall that as GFR increases, there is less time in the tubule to reabsorb Na and therefore less absorption of H20).
- So we don't want GFR to be changing all the time.
- There are two mechanisms by which GFR is regulated: myogenic and tubuloglomerular feedback.
- Myogenic GFR regulation
- This mechanism is not unique to the kidney; many vascular beds use it, including the brain.
- Recall that the point is to keep GFR at some constant levels and that an increased arterial blood pressure would increase GFR.
- So as arterial blood pressure increases, we want to myogenically decrease the blood pressure to maintain the same GFR.
- As arterial blood pressure increases, the vascular endothelial wall is stretched, stretch sensors on vascular smooth muscle cells open Ca channels, Ca enters the smooth muscle, muscle contracts, the lumen diameter decreases, and the vascular resistance increases.
- By this Stretch-Ca-based contraction of vascular smooth muscle and increased resistance, the renal blood flow (RBF) remains constant even when systemic blood pressure is elevated.
- Tubuloglomerular feedback
- Recall that the point is to keep GFR at some constant level because we don't want to lose too much Na or too much water (occurs when GFR is too high--not enough time in tubule to reabsorb the Na and H20).
- Note that there can be multiple afferent arterioles for a single glomerulus.
- The macula densa detects when NaCl levels in the filtrate are elevated.
- When filtrate NaCl levels are elevated, the macula densa cells release ATP which causes constriction of the afferent arterioles.
- Constriction of the afferent arterioles leads to decreased glomerular capillary hydrostatic pressure (PGC to that nephron) and therefore decreased GFR (in that nephron).
- Note that ATP is metabolized to adenosine in the interstitial fluid space between the macula densa and the smooth muscle cells of the afferent arteriole.
- Adenosine binds the A1 receptor on the afferent arteriole.
[edit] Renal sympathetic nerves and RBF control
- The two autoregulation control mechanisms for GFR are myogenic and tubuloglomerular; however, the body has a third for emergent situations: sympathetic nervous control.
- So, in these emergent situations, the blood pressure has drastically dropped for some very bad reason (trauma, et cetera).
- Sympathetic nervous control of the renal blood flow works by rapidly, temporarily constricting the afferent arterioles.
- This is a prioritization of water retention and continued blood flow to other organs over the proper function of the kidneys.
- Sympathetic nervous control is achieved through direct constriction, release of renin and release of catecholamines (epinephrine and norepinephrine).
- Direct innervation of the arterioles can cause constriction.
- Renin starts the angiotensin pathway which leads to angiotensin 2 and thus vasoconstriction, aldosterone release, and AVP release (all of which elevate blood pressure).
- Epinpehrine and norepi bind the a1-adrenoreceptors to directly cause vasoconstriction of the vascular smooth muscle.
[edit] Hormomal control of RBF
- In addition to autoregulation and sympathetic emergent control of RBF, there is long-term control via endogenous hormones.
- Renal vasodilators:
- Recall that vasodilation increases RBF, increases GFR, and increases loss of Na and H20.
- Prostaglandins, NO, dopamine, atrial natriuretic peptide
- Renal vasoconstrictors:
- Recall that vasoconstriction decreases RBF, decreases GFR, and decreases loss of Na and H20.
- Angiotensin 2, epi, norepi, throboxane A2, adenosine
- Thromboxane makes sense because it is activated when bleeding / clotting which is a good time to conserve water.
- Angiotensin 2 makes sense because it generally serves to conserve and reabsorb water, and decreasing RBF will slow filtrate flow and thus allow the tubule cells to reabsorb more of the H20.
[edit] The dampening effect of prostaglandins on renal vasoconstriction
- We have seen that sympathetic nerves cause vasoconstriction at the kidney (renin + epi / norepi -> vasoconstriction of the afferent arteriole).
- We have also seen that prostaglandins cause vasodilation at the kidney (vasodilation of the afferent arteriole).
- Finally, we know that NSAIDs decrease prostaglandin synthesis systemically.
- So, it makes sense that giving NSAIDs to a pt who is volume depleted (or otherwise has poor kidney function) is bad because it will reduce prostaglandin synthesis, therefore reduce the amount of vasodilation force on the afferent arteriole, and result in lower RBF, lower GFR, and less filtration.
- So, think of prostaglandins of the brake that slows vasoconstriction.
- "PG’s are always produced and act locally due to rapid destruction. The kidney produces its own PGs."
[edit] Hallmark of glomerular disease
- The hallmark of damage to the glomeruli is protein in the urine (proteinuria).
- Measuring protein in the urine underestimates the amount that is let into the filtrate at the glomerulus.
- This is because much of the filtered protein is metabolized or endocytized while it is part of the filtrate in the tubule.
[edit] Glomerular filtration occurs over 3 layers
- There are three cell types in the glomerulus:
- Endothelial cells of the capillaries
- Podocytes (visceral epithelial cells)
- Mesangial cells
- They hold stuff together
- Messangial cells are contractile; might be able to change filtration by covering up filtration slits or not.
- There are three major layers in the glomerulus through which a molecule must pass to get from the blood to the filtrate.
- The first layer is the capillary's endothelium.
- There are fenestrae through which most anything except cells can pass.
- However, we don't want proteins to pass through the endothelial barrier b/c they are large and will clog the glomerulus.
- The fenestrations of the endothelial cells are negatively charged to repel proteins (which are generally negatively charged).
- The fenestrae are about 70nm in diameter.
- The second filtration level is the basement membrane
- The endothelial cells of the arteriole sit on the basement membrane.
- The last specialization are the podocytes.
- Podocytes sit on the inside of the Bowman's capsule and send out feet from their cell body.
- The feet of neighboring podocytes rest very near to one another to form small slits through which only small molecules can pass.
- The slits formed by the podocytes are called filtration slits or slit pores.
- The filtration slits are about 4-14 nm in diameter.
- NEPHRIN is a critical structural protein for filtration slits.
- We believe NEPHRIN is a critical protein for proper filtration because when it is mutated, massive proteinuria occurs.
[edit] Factors affecting filterability
- Size, shape, deformability, and electrical charge are the major factors in deformability.
- Size: Albumin and hemoglobin rarely make it into the filtrate but myoglobin does readily enter the filtrate.
- Charge: albumin has a mass very similar to Hb but is found much less in the filtrate, probably due to albumin's negative charge.
[edit] GFR is determined by Starling forces
- Recall Starling forces which apply to all capillary beds of the body, including the renal capillaries and the glomeruli:
- There are four forces affecting flow from blood to interstitial fluid and vice versa.
- In the case of GFR, the competing fluids are capillary blood and the filtrate, not blood and interstitial fluid.
- Blood colloid pressure (PiGC) wants to keep stuff in the blood.
- Note that the blood colloid pressure (PGC) increases proximal to distal in the capillary as water is filtered out.
- Filtrate colloid pressure (PFC) wants to keep stuff in the interstitial fluid.
- Capillary hydrostatic pressure (PGC) wants to force stuff out of the capillary.
- Filtrate hydrostatic pressure (PBSwants to force fluid into the blood.
- PBS is negligible.
- There is a constant called the glomerular ultrafiltration coefficient (Kf) that accounts for the normal surface area and capillary permeability.
- Note that "ultrafiltration" is also the name for the overall filtration process that is occurring at the nephron.
- If there is vascular damage, the glomerular ultrafiltration coefficient may decrease.
- GFR = Kf * (PGC - PBS - PiGC)
- That is GFR = ultrafiltration coefficient * (capillary hydrostatic pressure - filtrate hydrostatic pressure - blood colloid pressure).
[edit] Force differences along systemic capillaries and renal capillaries determine GFR
- There is a distinct difference between systemic capillaries and the renal glomerular capillaries.
- Recall that systemic capillaries must pass nutrients from blood to tissue and pass waste from tissue to blood.
- This two-way exchange is facilitated by decreasing capillary hydrostatic pressure (over the distance of the capillary, the blood hydrostatic pressure decreases) and the static capillary colloid osmotic pressure (the amount of protein in the blood doesn't change as the blood passes through the capillary).
- At the proximal part of the systemic capillary, the hydrostatic pressure is greater than the blood colloid pressure so nutrients pass from the blood to the tissue.
- At the distal part of the systemic capillary, the hydrostatic pressure is less than the blood colloid pressure so wastes pas from the tissue to the blood.
- Recall that systemic capillaries must pass nutrients from blood to tissue and pass waste from tissue to blood.
- There are two separate sets of renal capillaries: the glomerular capillaries for generating filtrate and the renal peritubular capillaries for exchanging nutrients and wastes of the tubule cells.
- The glomerular capillaries function to generate filtrate and therefore do not have two-way exchange like the systemic capillaries:
- The capillary hydrostatic pressure (PGC) is much higher in glomerular capillaries than in systemic capillaries.
- Because the capillary hydrostatic pressure (PGC) is so much higher and changes so little from proximal to distal glomerular capillary, there is no point in the capillary where waste (filtrate) is brought into the capillary--the net force is always out of the blood at the glomerular capillaries.
[edit] Afferent and Efferent arteriole pressures and GFR
- The GFR can be controlled by changing the diameter of the afferent and efferent vessels.
- Recall that GFR = Kf * (PGC - PBS - PiGC)
- Note that the only variable in this equation that is dependent on the blood pressure is PGC.
- Afferent constriction
- Afferent constriction occurs when signaled by hormones (long term), sympathetics nerves (acute, via renin and epi), or via autoregulation (myogenic or tubuloglomerular).
- Upon constriction of the afferent arterioles there is decreased GFR because of decreased hydrostatic pressure PGC.
- Afferent dilation has the opposite effects: increased GFR and increased glomerular blood flow.
- Efferent constriction
- Efferent constriction occurs via even low angiotensin 2 levels.
- Upon constriction of the efferent arterioles there is increased GFR because of increased hydrostatic pressure PGC.
- In actuality, the GFR would go down if the degree of constriction is so severe that blood flow to the nephron is significantly reduced.
- Efferent dilation decreases the PGC and decreases GFR.
- It is important to recognize that the glomerular blood flow rate and the GFR are not directly related.
- See GC)_and_GFR two headings later
- It is the hydrostatic pressure of the capillary that determines the GFR.
- It is the pressure gradient of the afferent and efferent arterioles that determines the glomerular blood flow.
[edit] Bowman space pressure (PBS) and GFR
- Recall that the pressure of the Bowman space (PBS) opposes the hydrostatic pressure of the glomerular capillary blood.
- So, when PBS increases because of pathology, the GFR will decrease.
- Pathologies generally cause a backup or resistance in the tubule or ureter: kidney stones, prostatic hyperplasia, etc.
[edit] Blood colloid pressure (PiGC) and GFR
- When adding saline to a pt's blood, the colloid osmotic pressure of the blood at the glomerular capillary will decrease (fewer proteins per ml).
- Recall that GFR = Kf * (PGC - PBS - PiGC)
- So when PiGC (blood colloid osmotic pressure) decreases, GFR goes up.
- Does giving saline and therefore increasing GFR decrease mean that a higher dose or a more frequent administration of a drug must be given to be effective (because of increased clearance rate)?
- "makes sense – also depends on the stability/metabolism of the drug."
[edit] Relationship of glomerular blood flow and GFR
- The rate that blood flows through the capillaries of the glomerulus does affect how much filtration occurs.
- As blood flows through the glomerular capillary, stuff is lost to the filtrate but proteins (over 30kda) are not, thus blood colloidal osmotic pressure (COP = PiCG) increases from proximal to distal in the glomerular capillaries.
- This increase in COP (PiGC) is a function of the blood flow: the slower the blood flows the higher the COP of the blood.
- This makes because the longer the blood remains in the filtering area, the more filtrate will leave the blood (since hydrostatic pressure is forcing stuff out of the blood).
- PiGC (the colloidal osmotic pressure) will rise until it is high enough to oppose the hydrostatic pressure (that is, until the sum of PGC and PBS is equal to PGC).
- When blood flow is too low, the equilibrium of PGC and (PBS</sub + PiGC) occurs quickly and not all the surface area of the capillaries is used for filtration, which is bad because decreased filtration is like, well, kidney failure.
[edit] Normal GFRs
- Normal GFRs change with age and gender.
- Neonates: 20 ml / min / 1.73 m2
- Young adult, female: 110 +/- 15 ml / min / 1.73 m2
- Young adult, male: 125 +/- 15 ml / min / 1.73 m2
- GFR declines after 45.
- GFR is 30-40 lower at age 80 than 21.
- In healthy, young adults, the GFR is high primarily because:
- Kf is high (there is a large surface area and there are many pores in the capillaries)
- PGC is high (blood pressure is as high as it should be, not higher, not lower)
- Glomerular blood flow is high (which results in a low PiGC and therefore less counterforce to the hydrostatic pressure).
[edit] GFR is an important metric
- GFR is an important measure of renal function.
- Higher GFR at 12 months post-transplant is a good predictor of graft survival for 10 years.
- stopped here on 03/23/11.
- started here on 03/23/11
[edit] Tubular reabsorption and secretion
[edit] Three processes involved in urine formation
- There are three major processes involved in forming urine: filtration, reabsorption, and secretion.
- Filtration occurs at the glomerulus and is the movement of material from the blood to the filtrate
- See Renal blood flow, glomerular filtration for more information
- Reabsoprtion occurs at the tubule and is the movement of desired material from the filtrate into the ECF.
- Secretion occurs at the tubule and is the movement of waste material from the ECF to the filtrate.
- Filtration occurs at the glomerulus and is the movement of material from the blood to the filtrate
[edit] Calculating the reabsorption of a solute
- We can calculate how much of a solute is reabsorbed.
- We start with the filtered load: the amount presented to the tubule / min.
- Treabs = Px * GFR - Ux * V, where:
- Px is the plasma concentration of the substance (x) (mg / ml)
- GFR is the glomerular filtration rate (ml / min)
- Ux is the urinary concentration of the substance (x) (mg / ml)
- V is the urine flow rate (ml / min, not the volume!)\
- The units of Treabs (the reabsorption rate) will come out to mg / ml / min.
[edit] Calculating the secretion of a solute
- We can calculate how much a substance is secreted at the tubule.
- As with the reabsorption rate, we start with the filtered load: the amount of a substance presented to the tubule (mg / min).
- Then Tsecr = Ux * V - Px * GFR, where:
- Ux is the urinary concentration of the substance x (mg / ml)
- V is the urinary flow rate (ml / min)
- Px is the plasma concentration of the substance x (mg / ml)
- GFR is the glomerular flow rate (ml / min)
- The units of Tsecr (the secretion rate) will come out to be mg / ml / min.
[edit] Fractional excretion differentiates between reabsorption or secretion
- Notice that the variables of measuring Treabs and Tsecr are the same, we simply reverse the order of subtraction.
- These two terms ("Px * GFR" and "Ux * V") have their own names: filtered load and urinary excretion.
- Recall that the filtered load of substance x is the amount seen by the tubule and is defined as Px * GFR (which results in a mg/min term).
- So the filtered load describes how much of the substance enters the tubule / minute.
- Recall that the urinary excretion of substance x is the mount secreted and is defined as Ux * V (which results in a mg/min term).
- So the urinary excretion describes how much of the substance exits the tubule / minute.
- When we ask if a substance is net reabsorbed or net secreted we are asking if the filtered load is greater or the urinary excretion is greater.
- The ratio (fraction) of the amount of the substance that enters the tubule (filtered load) to the amount that exits the tubule (urinary excretion) tells us whether the substance was reabsorbed or secreted or neither.
- When the ratio (of filtered load to urinary excretion) is over 1, the substance is reabsorbed (which makes sense because more entered the tubule than left the tubule so there must have been some reabsorption).
- When the ratio is 1, the substance is secreted and reabsorbed equally.
- when the ratio (of filtered load: Px * GFR, to urinary excretion: Ux * V) is less than 1, the substance is secreted (which makes sense because less is filtered than is excreted so there must have been some tubular secretion).
- The ratio (fraction) of the amount of the substance that enters the tubule (filtered load) to the amount that exits the tubule (urinary excretion) tells us whether the substance was reabsorbed or secreted or neither.
- So what is the FE for inulin?
- We know that inulin shows little reabsorption so the filtered load will be equal to the urinary excretion.
- The ratio will be 1:1; the fraction will be 1.
- What is the FE for glucose?
- We know that glucose is reabsorbed very well in the tubule so the filtered load will be much higher than the urinary excretion.
- The ratio will be 100:1 (as an example); the fraction will be 100 (as an example).
[edit] Glucose reabsorption
- Glucose reabsorption takes place in the renal proximal tubule.
- Glucose reabsorption (from the lumen into the proximal tubule cell) occurs through a Na-Glucose cotransporter (SGLT) on the apical surface of the proximal tubule cell by way of the Na gradient generated by Na / K ATPase on the basal surface of the proximal tubule cell.
- Second, glucose is moved from the proximal tubule cell to the ECF / blood by facilitated diffusion by way of a GLUT protein.
[edit] Why does glucose reach a maximal Treabs?
- Recall that doctors of old used to taste the urine of patients to diagnose diabetes; the glucose levels of a diabetic patient can be so high that the tubule cannot reabsorb it all making the urine taste sweet.
- At normal levels, glucose enters the filtrate but is very well reabsorbed such that urinary excretion (Ux * V) values are very low.
- When glucose reaches a very high level in the blood, the epithelial cells of the tubule don't have enough time to reabsorb all the glucose in the filtrate (glycosuria).
- Recall that reabsorption of glucose occurs in the proximal tubule via SGLT (active) and then GLUT (passive).
- Because not all the glucose can be reabsorbed at these high levels, there is increased osmotic pressure in the filtrate and less water is reabsorbed causing polyuria.
- There is a difference in the maximum glucose reabsorption rate in cortical and juxtamedullary glomeruli.
[edit] Micropuncture and microperfusion of nephrons in vivo
- A nephron can be punctured and perfused with well handled micropipettes; this allows for experimental determination of nephron function.
- For example, we can sample filtrate along the tubule and measure the concentration of inulin at each location.
- Concentrations of inulin increase as one travels distally.
- Since we know that inulin doesn't get reabsorbed, we can determine that the concentration is increasing because water is being reabsorbed.
- We can also measure the concentration of inulin in the tubular fluid and in the plasma.
- The ratio of the tubular fluid concentration to plasma concentration is a function of the length of the nephron: the farther along the nephron, the higher the ratio; that is, the farther along the nephron, the more inulin found in the filtrate and the less found in the plasma.
- The huge change in concentration between the DCT and the urine represents that ability of the collecting duct to significantly concentrate the filtrate even in its already-pretty-concentrated form.
- This graph also shows us that the PCT is specifically designed for and is very capable of bulk reabsorption of solutes and water.
- This is evidenced by the rise in ratio over a short distance.
[edit] Proximal tubule fluid is essentially iso-osmotic to plasma
- In general, water is reabsorbed along the tubule by way of an osmotic gradient between the ECF and the filtrate.
- This reabsorption occurs through the cells via aquaporin channels (AQP).
- Recall that ADH causes increased expression of AQP (aquaporin) at the distal convoluted tubule and the collecting duct, thus increasing water reabsorption (and increasing blood pressure).
- This reabsorption occurs through the cells via aquaporin channels (AQP).
- In the proximal tubule, there is no such osmotic gradient between the ECF and the filtrate.
- This means the proximal tubule is not responsible for much water reabsorption.
- There is no gradient because of the high water permeability of the epithelium.
- We can observe how the body acts to conserve water by measuring the ratio of urine and plasma osmolarity when rats are infused with waste molecules:
- Inducing water loss by administering lots of a waste product is called diuresis as in "mannitol diuresis".
- When urea, glucose, saline, and mannitol are given, the osmolarity of urine rises more rapidly than plasma (causing the U / P ratio to increase); this indicates the ability of the kidney to concentrate urine.
- The highest urine osmolarity to plasma osmolarity (U / P ratio) is seen when rats are dehydrated.
- Note that while many solutes and water molecules are being reabsorbed in the PCT, the osmolarity of the filtrate and plasma are similar throughout the PCT.
- This indicates that the PCT is capable of bulk transport but not of concentrating the filtrate (because then the osmolarity would increase).
- Recall that diabetes insipidus occurs when ADH is elevated such that water loss is large.
[edit] Perfusion of isolated tubules can teach us about the PCT
- PCT = proximal convoluted tubule.
- Experimentally, we can perfuse a short segment of a tubule and collect the filtrate at the other end.
- This allows us to measure what is being reabsorbed and secreted in this small area.
- Note that in perfusion / puncture studies we expect reabsorbed things to decrease in TF / P ratio and secreted molecules to increase in TF / P ratio.
- This makes sense because....
- Through these puncture studies we have learned about the PCT:
- Glucose and aas are nearly 100% reabsorbed in the PCT.
- Na, H20, and K are 70% reabsorbed in the PCT.
- HCO3- and Cl- accompany Na reabsorption in the PCT.
- HCO3- transport is favored over Cl transport.
- It makes sense that HCO3- is reabsorbed over Cl- in the PCT because Cl- can be reabsorbed with Na later in the tubule (ascending loop and distal convoluted tubule).
- Urea is 50% reabsorbed in the PCT.
- Inulin becomes more concentrated in the PCT.
- This means that water is reabsorbed in the PCT.
- This provides a measure of how much water is reabsorbed.
- Organic ions (like PAH) are secreted.
- Recall that PAH is secreted "so vigorously" that nearly all of it is removed from the blood in a single pass.
- Because nearly all PAH is removed in a single pass, PAH is a good indicator of renal plasma flow (when Hct is taken into account).
- Note that PCT filtrate remains iso-osmotic (relative to plasma) because there is lots of water permeability.
- That is, while lots of glucose, aas, Na, K, and urea are being reabsorbed so you might think the urine would be starting to concentrate, in actuality, water is following the many of these molecules into the blood (because the epithelium is leaky) so the osmolarity of the filtrate and plasma remain about the same.
[edit] Na reabsorption in the PCT
- Recall that most of the oxygen used by the kidneys goes to generating ATP for the Na / K ATPase.
- The consistent, large Na gradient allows tubule cells to couple transport of many molecules to Na.
- Na reabsorption is the main driving force for reabsorption of solutes (like glucose and aa) and water.
- Recall that the epithelium of the PCT is leaky, so as solutes are moved via Na gradient, H20 can and will follow.
- Recall that glucose is moved via the Na-Glucose symporter SGLT on the apical surface of the tubule cells and then via the passive GLUT transporter on the basal membrane.
- Na is also moved via Na-aa channels on the apical surface.
- Note that K+ does not accumulate in the epithelial cell (from Na / K ATPase activity on the basal surface) because of K+ ions pores on the basal surface.
[edit] Reabsorption: from tubular cell to blood
- The Na gradient produced by the tubular cell and Na / K ATPase moves solutes and water into the cell's cytoplasm.
- Mostly passive movement of solutes and water down their concentration gradients moves them from the tubular cell into the interstitial fluid between the tubular cells and the endothelial cells of the peritubular capillaries.
- Recall that there are two sets of capillaries in the kidney: glomerular and peritubular.
- Movement of these reabsorbed solutes and water molecules from the ICF (intercellular fluid) to the blood is (of course) determined by the four Starling forces:
- Hydrostatic pressure of the blood and colloid osmotic pressure of the ICF force solutes to stay in the ICF.
- The hydrostatic pressure of the blood in the peritubular capillaries is much lower than it was at the glomerular capillaries.
- The plasma colloid pressure is high because so much fluid was lost at the glomerulus yet the proteins remained.
- Hydrostatic pressure of the ICF and colloid osmotic pressure of the blood force solutes to move into the blood.
- Interstitial hydrostatic pressure is increased because of the active pumping of solutes with water following.
- Hydrostatic pressure of the blood and colloid osmotic pressure of the ICF force solutes to stay in the ICF.
[edit] PCT and secretion of organic ions
- Recall that the PCT is responsible for secretion of organic ions in addition to reabsorption of glucose, aa, Na, K, Cl, and HCO3.
- Secreting organic ions is a two step process: the organics must get through the basal and apical membranes of the tubule epithelial cell.
- Anions must be accompanied by carrier proteins as they cross the tubular cells (from basal to apical surface).
- This is one reason for drug interactions: when one drug (anion) preferentially binds the carrier protein, another drug (anion) may be not be secreted as fast, causing an elevated effect at a normal dosage.
- OAT1 (organic anion transporter) and OCT (organic cation transporter) are two important transport proteins for ions and are found on the basal membrane of the tubular cells.
- OAT1 is the transporter that so effectively secretes PAH.
- Here are some organic anions secreted at the PCT:
- Phenol red (a pH indicator dye)
- PAH (used for measurement of renal plasma flow)
- Penicillin (an antibiotic)
- Probenecid = benemid (inhibits penecillin secretion, inhibits uric acid reabsorption)
- Was important back when penecillin was so expensive and we wanted to keep it in the pt's blood.
- Furosemide = lasix (a loop diuretic drug)
- "Loop diuretics act on the Na+-K+-2Cl- symporter (cotransporter) in the thick ascending limb of the loop of Henle to inhibit sodium and chloride reabsorption. This is achieved by competing for the Cl- binding site." per wikipedia
- Acetazolamide = Diamox (Carbonic anhydrase inhibitor)
- Recall that carbonic anhydrase converts between CO2+H20 and H + HCO3, thus controlling blood pH.
- Creatinine (normal end product of muscle metabolism)
- Here are some organic cations secreted at the PCT:
- Histamine (vasodilator, stimulator of gastric acid secretion)
- Cimetidine (drug for treatment of gastric and duodenal ulcers)
- Cisplatin (cancer therapy drug)
- Norepi (neurotransmitter)
- Quinine (antimalarial drug)
- Tetraethylammonium = TEA (ganglion blocking drug)
- Creatinine (normal end product of muscle metabolism; models GFR)
[edit] Excretion of lipid soluble organics
- Lipid soluble organics get into the filtrate through non-ionic diffusion through the tubular cell membranes.
- The lipid soluble molecules would simply diffuse back out if they are not trapped in the filtrate.
- The tubule cells pump hydrogen (H+) into the filtrate in order to trap these lipid soluble molecules in the filtrate.
- The idea here is that neutral, polar solutes will not remain in the filtrate, so the filtrate can be acidified (with H+) or alkalized (with HCO3-) to cause lipid-soluble substrates to adopt a charged or non-polar state so they will remain in the filtrate.
- For example, H+ reacts with ammonia to trap it in the filtrate.
- H+ reacts with acids to neutralize and reabsorb them into the blood.
- The idea here is that neutral, polar solutes will not remain in the filtrate, so the filtrate can be acidified (with H+) or alkalized (with HCO3-) to cause lipid-soluble substrates to adopt a charged or non-polar state so they will remain in the filtrate.
- Phenobarbital is used as a sedative and is a lipid-soluble weak oranic acid (A-).
- In order to neutralize phenobarbital when you no longer want your patient sedated, you give NaHCO3-.
- NaHCO3- dissociates into Na and HCO3- and increases the HCO3- concentration of the blood.
- Increased plasma HCO3- will result in less reabsorption of HCO3- in the proximal tubule and thus an increased alkalinity of urine.
- At higher alkalinity (that is, fewer H+), phenobarbital will remain as an acid (and not bind H+) in the filtrate and thus be secreted (instead of binding H+, neutralizing, and being reabsorbed).
[edit] Loop of Henle
- The loop of Henle serves to dilute the urine.
- Dilution occurs because the ascending loop is impermeable to water (that is, water from the filtrate cannot be reabsorbed) yet there is active reabsorption of solutes like Na and Cl.
- Thus, as the epithelial cells remove solutes but leave behind the water that would like to follow, the filtrate becomes more dilute.
- The loop of Henle is where loop diuretics work.
- Loop diuretics cause a loss of water and are therefore useful in treating hypertension (by decreasing the extracellular fluid compartment).
- Loop diuretics include furosemide, bumetanide, etc.
- Loop diuretics inhibit the Na / K / 2Cl cotransporter of the ascending limb that moves Na, K, and Cl from the filtrate to the interstitial fluid.
- One might think that this means water will not follow, but recall that the ascending limb is nearly impermeable to water anyhow.
- Thought loop diuretics have their pharmacological effect on the channels and cells of the ascending loop, loop diuretics have their physiological affect on the collecting duct.
- When loop diuretics decrease the Na / K / 2Cl content of the interstitial fluid between the (parallel) ascending loop and collecting duct, water reabsorption is decreased at the collecting duct because there is less osmotic force pulling water from the filtrate to the interstitial fluid.
[edit] Distal terminology
- To describe physiological functions, we talk about the "distal tubule" and the "distal nephron".
- The distal tubule is more than just the convoluted tubule (DCT).
- The distal tubule includes the distal convoluted tubule, the connecting tubule, and the initial cortical collecting duct.
- The distal nephron is the distal tubule plus all of the collecting duct.
- The distal nephrn is the DCT, connecting tubule, cortical collecting duct, outer medullary collecting duct, and inner medullary collecting duct.
[edit] Thiazide diuretics
- Thiazide diuretics cause a decrease in water reabsorption and are thus useful for treating hypertension (by decreasing volume of the extracellular compartment).
- Thiazides include chlorothiazide and metolazone.
- Thiazides inhibit the Na / Cl cotransporter at the distal convolunted tubule (DCT) causing a decrease in Na / Cl reabsorption.
- Decreased reabsorption means there is more Na and Cl in the filtrate; that is, the filtrate will be less dilute.
- Recall that the job of the collecting duct is to reabsorb water and that the collecting duct achieves water reabsorption by the osmotic gradient between the medullary area and the filtrate.
- So when solutes of the filtrate are not reabsorbed, the osmotic pull of the filtrate increases and there is a smaller difference in the medullary and filtrate osmotic forces, resulting in decreased water reabsorption.
- More explicitly, when thiazide diuretics decrease Na / Cl reabsorption, they increase filtrate osmotic force, they decrease the medulla-filtrate osmotic gradient, and they decrease water reabsorption at the collecting duct.
- Note that loop diuretics decrease solute reabsorption at the ascending loop and thiazides decrease reabsorption at the DCT, but both loop diuretics and thiazides achieve their physiological effect by decreasing water reabsorption at the collecting duct.
[edit] Collecting tubule
- The collecting tubule is the last segment of the nephron.
- The collecting tubule is a tight epithelium and contains two cell types: principle cells and intercalated cells.
- 2/3 of the cells of the collecting duct are principal cells and 1/3 of the collecting duct cells are intercalated cells.
- The collecting duct affects Na, K, and H+ secretion / reabsorption.
- Principal cells help the rest of the epithelial cells of the nephron regulate Na, K, and water.
- Principle cells reabsorb Na and water while excreting K.
- K secretion make sense because the Na / K ATPase on the basal surface of the principal epithelial cell generates a flow of Na into the blood (reabsorption, which H20 follows) and a flow of K out into the filtrate.
- "K+ secretion is increased when urine flow increased due to diuretic action (problem of K+ wasting)."f
- That is, diuretics increase the flow of filtrate (by inhibiting reabsorption in some way) and because K flows from the tubular cells to the filtrate passively, the higher the flow rate the more depleted the cells will be of K.
- Thus, more K is lost when the flow rate is faster.
- "Any diuretic drug will increase the flow rate of tubular fluid because the drug ultimately inhibits water reabsorption. When increased flow reaches the collecting duct is can lead to potassium wasting. This occurs because K is secreted in the CD by a passive process driven by high intracellular K ( in principal cells). High flow rate immediately sweeps away any secreted K and therefore maximizes the concentration difference so K secretion will be maximal and higher than normal. Can lead to hypokalemia so a real problem with some diuretics."
- Intercalated cells help maintain pH homeostasis.
- There are two types of intercalated cells: one to move the pH up (alkalinate) and one to move the pH downward (acidify).
- Intercalated cells function to maintain blood pH by secreting what is high and reabsorbing what is low.
- The only difference in intercalated cells is which of H+ and HCO3- they secrete and which they absorb.
- Alpha cells (think "a for aaaahh! too much acid") secrete H+ and reabsorb HCO3-, thus raising the pH of the blood.
- Alpha cells secrete H+ via an apical H+ATPase and an apical H+/K+ ATPase.
- AE1 is the protein of alpha cells that exchanges HCO3- into the blood for Cl- into the alpha intercalated cell.
- Note that H+ secretion is an active process taking place on the apical surface of alpha intercalated cells of the collecting duct.
- Beta cells secrete HCO3- and reabsorb H+, thus decreasing the pH of the blood.
- Pendrin is the protein of the beta cells that exchanges HCO3- into the lumen for Cl- into the beta intercalated cell.
- Movement of H+ (in either direction: secretion or reabsorption) is achieved by antiporting with K+.
- Alpha cells secrete H+ into the filtrate and thus reabsorb K+ (because to move H+ into the filtrate we have move K+ into the blood).
- Therefore alpha intercalated cells are activated when dietary K+ is low such that K+ reabsorption becomes a high priority.
- AE1 and pendrin are both HCO3- / Cl- exchangers; AE1 is used by alpha cells to move HCO3 into the blood while pendrin is used by beta cells to move HCO3 into the filtrate.
- Note that via intercalated cells of the collecting duct, the kidney can help regulate blood pH.
[edit] Aldosterone's effects on the kidney
- Aldosterone acts on the principal and intercalated cells of the collecting duct.
- Recall that aldosterone is released from the zona glomerulosa of the adrenal gland in response to angiotensin 2 signaling.
- Aldosterone acts on the principal cells in the collecting duct of the nephron to increase water reabsorption.
- Aldosterone increases expression of ENaC on the apical surface.
- Aldosterone also increases the expression of Na / K ATPase on the basal surface of the principal cells.
- Both of these increases in protein expression / function will cause an increase in Na / water reabsorption from the filtrate.
- Aldosterone acts on the intercalated cells of the collecting dcut of the nephron to decrease blood pH.
- Aldosterone increases alpha intercalated cell activity which causes increased secretion of HCO3- into the blood and H+ into the filtrate.
- Amiloride is an anti-ENaC drug that is used to decrease Na / H20 reabsorption and thus decrease water retention and blood pressure.
- Recall that ENaC is used by epithelium of the collecuting tuble.
- Recall that aldosterone causes increased ENaC expression on the principal cells of the collecting duct.
[edit] ADH's effects on the kidney
- ADH affects principal cells of the collecting duct (as well as epithelial cells of the DCT).
- Recall that ADH is released from the posterior pituitary upon sensation of low blood pressure at the hypothalamus.
- ADH causes increased aquaporin-2 on the apical surface of the DCT and collecting duct epithelial cells.
- Recall that aquaporin-2 is a water channel.
- ADH binds the g-coupled V2 receptor which causes and increase in cAMP, an increase in Ca, and PKA activation which all lead to increased aquaporin-2 expression and exocytosis of vesicles holding aquaporin-2 channels.
[edit] Potassium sparing diuretics
- Potassium-sparing diuretics cause a decrease in water reabsorption and are thus useful for treating hypertension (as they decrease the volume of the extracellular compartment).
- Recall that principal cells of the collecting duct reabsorb Na and water while secreting K.
- Potassium-sparing diuretics act on the ENaC channels of principal cells to decrease Na reabsorption through ENaC.
- It makes sense that these diuretics are called "potassium-sparing" because they will allow the body to keep its potassium stores; when you inhibit the flow of Na from filtrate to blood, you will also arrest the flow of K from blood to filtrate (because of electrochemical gradients across the epithelial principal cell).
- Note that potassium-sparing diuretics act on cells of the collecting tubule and have their physiological effect at the collecting tubule.
[edit] Comparison of PCT and Collecting duct
- The PCT and DCT differ in their inherent water permeability, precision of reabsorption control, transport capacity, and transepithelial gradients.
- Inherent water permeability:
- The PCT's inherent water permeability is high while the collecting duct's is low.
- Recall that the PCT reabsorbs most of the solutes and water of the filtrate.
- Transport capacity:
- The PCT has a very high transport capacity while the collecting duct's transport capacity is smaller.
- Recall that the PCT reabsorbs most of the solutes and water of the filtrate.
- Precision of reabsorption control:
- The PCT's control is course while the collecting duct's control is fine.
- Recall that diuretics, ADH, and aldosterone all work at the collecting duct (or at least the latter parts of the nephron).
- Transepithelial gradient
- The PCT has a low transepithelial gradient while the collecting duct has a high transepithelial gradent.
- Recall that the DCT generates a high transepithelial gradient so the collecting duct can reabsorb lots of water.
- Water and Na reabsorption by percent and nephron location:
- Na reabsorption:
- PCT: 70%
- Loop of Henle: 20%
- DCT and CD: 9%
- Water reabsorption:
- PCT: 70%
- Loop of Henle: 10%
- DCT and CD: 19%
- Na reabsorption:
[edit] Inherited defects in kidney tubule epithelial cells
- There are many diseases that affect the epithelial cells of the kidney tubule:
- Renal glucosuria (SGLT – Na / Glucose cotransporter)
- A faulty SGLT protein would caused decreased Na / Glucose reabsorption, increased filtrate osmolarity, and decreased water reabsorption, thus causing increased urination and water loss.
- Cystinuria (amino acid transporter)
- A faulty amino acid transporter would caused decreased aa reabsorption, increased filtrate osmolarity, and decreased water reabsorption, thus causing increased urination and water loss.
- Can also cause kidney stones.
- Bartter syndrome (Na / K / 2Cl cotransporter)
- ...see above
- Gitelman syndrome (Na / Cl cotransporter)
- ...see above
- Liddle syndrome (ENaC)
- Severe hypertension
- ...see above
- Nephrogenic diabetes insipidus (V2 receptor or AQP2)
- A defective V2 receptor would cause decreased ADH signaling, decreased AQ2 (aquaporin-2) protein expression / release on principal cells, and decreased water reabsorption.
- Nephrogenic syndrome of inappropriate antidiuresis (increased V2 receptor activity)
- Increased V2 expression would cause an elevated response to ADH at the principal cells, and increased expression / release of AQ2 and increased water reabsorption.
- Renal glucosuria (SGLT – Na / Glucose cotransporter)
- started here on 03/24/11.
[edit] Water balance
[edit] Collecting duct review
- Recall what we learned about the collecting duct:
- 2/3 principal cells, 1/3 intercalated cells
- The collecting duct is a tight epithelium
- ENaC is expressed on the principal cells of the collecting duct and is used to reabsorb Na via loss of K.
- ENaC is also inhibited by amiloride.
- Amiloride is a potassium-sparing diuretic because it inhibits reabsorption of Na via ENaC and therefore inhibits the loss of K.
- Amiloride acts at the very end of the nephron (at the collecting duct on the ENaC channels), so it wastes less potassium.
- Recall that it is the principal cells where AVP works (it causes AQP to be expressed on the apical surface).
- It is also the principal cells where aldosterone work: increases expression of ENaC on the apical surface.
- Mutations in Liddle syndrome cause a slow down in turnover of the ENaC channels such that increased water reabsorption occurs and hypertension results.
- So, even at the very distant site of the collecting duct, we can still reabsorb enough Na to cause hypertension.
[edit] Water distribution in the body
- The amount of water as a percent of weight is dependent on age, gender, and amount of adipose tissue.
- Age generally decreases water as percent of weight.
- This is primarily due to the loss of muscle which is a high-water tissue.
- Women generally have less percent body weight in water than men.
- Increased adipose tissue leads to decreased percent of body weight as water.
- This makes sense because adipose tissue won't accomadate much water, so when more of your body weight is made of an anti-water material, you'll have less of your body weight percent be from water.
- Age generally decreases water as percent of weight.
- There are two major compartments for body fluids: intracellular (within cells) and extracellular.
- The intracellular compartment contains 40% (28 liters) of the body weight.
- The extracellular compartment contains a total of 20% (14 liters) of the body weight.
- Within the extracellular compartment there are two other compartments: interstitial water, plasma water.
- The interstitial water compartment contains 15% (10.5 liters) of the body weight.
- The plasma water compartment contains 5% (3.5 liters) of the body weight.
- Note how small the plasma water compartment is, relative to the interstitial and intracellular compartments.
- For women, only 50% of the body weight is made up of water and the intracellular compartment makes up 30% of the body weight.
- That is, women are less water by weight and the water they do have lies more heavily in the intracellular compartment.
- Women have a higher percent of adipose tissue, so it makes sense that they have less water as percent of body weight.
[edit] Effects of disturbances on osmolalities and volumes of ICF and ECF
- In a healthy, normal state, 28L of the body's water is in the intracellular compartment and 16 liters is in the extracellular compartment.
- In response to water added intravenously, osmolality of the ECF compartment decreases, then osmolality of the ICF will decrease to match the osmolality of the ECF, finally both intracellular and extracellular volumes will be increased in volume proportionally.
- In response to isotonic saline added intravenously, the extracellular compartment will expand but the intracellular compartment will remain fixed.
- Recall that saline is Na, Cl and water and that Na will not enter the intracellular compartment because of Na / K ATPase.
- Recall that water follows solute.
- Since Na will not leave the extracellular compartment, nor will water; thus the ECF compartment expands but the intracellular compartment does not.
- In response to 5% NaCl solution (that is, a Na Cl solution more concentrated than plasma) added intravenously, the ECF compartment will increase in volume (as water flows out of the intercellular compartment, into the ECF) and both compartments will increase in osmolality.
- Recall that 5% NaCl is a higher osmolality than normal blood and thus the Na and Cl of the solution will increase the osmolality of the ECF.
- Know how each of these is corrected the kidney.
- Think about hydrostatic pressures, ADH signaling, aldosterone signaling, etc.
[edit] Daily water balance in an average 70kg man
- The normal input of water is 2.5 liters from:
- 1 liter from beverages
- 1.2 liters from food
- 0.3 liters from water oxidation
- The normal output of water is 2.5 liters from:
- 0.9 liters from skin and lungs
- 0.1 liters from feces
- 1.5 liters from urine
- Note that the kidneys fluctuate their function to regulate output to match input.
- Recommendations for eight 8 ounce glasses of water each day are unfounded.
- There are good reasons to drink water, but 64 ounces is not an evidence-based rule.
- Water helps reduce stone formation in at-risk populations.
- Water helps maintain tooth health by increasing saliva flow.
- One should simply drink when thirsty and enough to generate about 1.5 liters of urine each day.
[edit] Thirst
- There are several mechanisms by which the sensation of thirst is generated.
- Thirst is generated by stimulating the hypothalamus.
- The hypothalamic response to thirst is to release ADH
- Recall that ADH arises from the hypothalamus, is released by the posterior pituitary, and causes insertion of aquaporin proteins on the apical surface of principal cells of the DCT and collecting duct of the nephron.
- Thirst is felt at any water loss greater than 3% of total body weight.
- An increase in plasma osmolality can generate thirst:
- There are two separate groups of osmoreceptors in the CNS: one set in the CNS that generates thirst sensation and another in the hypothalamus that generates ADH release.
- Osmoreceptors detect changes in the osmolality of the ECF based on how water is flowing across their own cell membrane.
- When water is flowing outward, the ECF is less dilute (higher osmolality) than the intracellular compartment.
- It is when water flows out of the osmoreceptors that these two groups of osmoreceptors generate their effects: thirst and AVP release.
- The osmoreceptors of the hypothalamus are neurons that project to other, larger neurons of the hypothalamus that produce AVP.
- Upon changes in water flow over the osmoreceptor, it signals to the larger neurons, which deliver AVP to the posterior pituity, and release AVP into the blood at the posterior pituitary.
- Note that this is a neuroendocrine system.
- A decrease in blood volume can generate thirst:
- Baroreceptors reside in the large vessels of the body and detect changes in blood pressure which can be indicative of blood volume.
- There are two types of baroreceptors: high pressure baroreceptors (cardiopulmonary) and low pressure baroreceptors (volume receptors).
- In regards to thirst, we are discussing the low pressure baroreceptors.
- Low pressure baroreceptors are found in the large veins, the pulmonary vessels, and the atrial walls.
- Volume baroreceptors use both innervation and hormones to correct volume changes.
- Volume baroreceptors (low pressure baroreceptors) use an inhibitory signaling to the hypothalamus to manage thirst and blood volume.
- That is, when denervated, the body things the blood pressure is low and tries to retain water.
- Low pressure baroreceptors release renin in response to low pressure and thus cause system-wide vasoconstriction and increased blood pressure.
Do these baroreceptors actually release renin or only signal for renin release?
- If the blood volume decreases by 10%, baroreceptors decrease their firing rate to the hypothalamus and increase renin release.
- Mouth and throat dryness can generate thirst:
- Innervation of the mouth and throat can cause the hypothalamus to experience thirst and respond with ADH.
- The GI tract can decrease thirst:
- As the stomach is distended or water is absorbed, the GI tract sends thirst-inhibiting nerve signals to the hypothalamus.
[edit] AVP
- AVP = arginine vasopressin = ADH = antidiuretic hormone
- AVP is encoded by an mRNA for a translation product called preproneurophysin.
- Preproneurophysin is made up of the AVP prohormone, neurophysin, and a glycopeptide.
- AVP (the active hormone) is only 9 aa long and has a plasma half-life of 9 minutes because of metabolism by the kidney and liver.
- Recall that one of the kidney's jobs is to destroy all the peptide hormones.
- We call AVP arginine vasopressin because of the arginine at the 8th position.
- Some other mammals like pigs, hippos, and some marsupials use lysine vasopressin with lysine at the 8th position.
- Lysine vasopressin can be used in human therapy, too.
[edit] AVP release
- AVP is released when osmolality of the ECF increases.
- The AVP release system is more sensitive to increases in plasma osmolality when the volume contracted.
- The opposite also remains true; the AVP release system is less sensitive to plasma osmolality changes when the ECF volume is elevated.
This makes sense, but how? Is it a function of signaling threshold at the hypothalamus where APs from both osmoreceptors and baroreceptors hit threshold more often than when only received from osmoreceptors?
- Osmolality is the more acute signal than blood pressure:
- AVP is released when plasma osmolality increases by only 1%.
- AVP is released when circulating volume decreases by 5-10%.
- Note that a normal plasma osmolality is 285-290 mOsm.
- ADH release occurs at around 280 mOsm.
- So we have a normal, small amount of AVP in the blood most of the time.
[edit] Factors that increase ADH release
- There are several factors that will increase the release of ADH in the body, several of which we have mentioned.
- Cellular dehydration will cause an effective increase in plasma osmolality and thus increase ADH release.
- High salt diets can cause cellular dehydration.
What causes cellular dehydration? What causes cellular dehydration without a proportional dehydration of the ECF? Does high salt diet really cause cellular dehydration?
- Hypovolemia will result in an effective decrease in arterial blood volume and increased signaling from baroreceptors.
- Pain, trauma, emotional stress, nausea, fainting, nicotine, morphine, angiotensin 2, and most anesthetics will increase ADH release.
- Recall that one of the body's responses to stress is to retain water.
- Recall SIADH (syndrome of inappropriate ADH secretion) which aberrantly increases ADH release and therefore increase water retention.
- There are also ways to decrease ADH release:
- Ethanol: recall that after alcohol intake, one feels increased urge to pee.
- Atrial natriuretic: recall that atrial natriuretic peptide is released when the atria detect high blood pressure, thus decreasing the stimulus to the kidney to retain water.
- Naturesis = loss of Na at the kidney.
[edit] AVP threshold versus thirst threshold
- The threshold for AVP release is about 280 mOsm.
- Humans generally demonstrate a plasma osmolality of about 285 mOsm, meaning AVP is released at some baseline level.
- Thirst, however, does not manifest until over 290 mOsm such that we do not feel the sensation of thirst all the time.
[edit] Urine osmolality versus plasma osmolality
- In a healthy state, urine has a higher osmolality than plasma.
- It makes sense that urine has a higher osmolality than plasma because we reabsorb so much of the water from urine but leave so many of the solutes.
- As AVP secretion increases, urine osmolality increases.
- That is, as AVP increases, water reabsorption increases and urine solute : water ratio goes up.
- We can concentrate urine to a maximum of about 1200 mOsm.
- At 1200 mOsm, increasing AVP does not cause increased concentration of the urine.
- When AVP release is very, very low, urine osmolality is less than plasma osmolality.
- What happens to AVP release when:
- The weather is cold: blood is shifted away from the skin, which is interpreted by the baroreceptors as an increase in volume and thus causes a decrease in AVP release and an increase in urine production.
- Swimming: the bouyancy provided by swimming allows the blood that usually sits down in the legs to distribute more evenly throughout the body. Thus, baroreceptors detect higher blood pressure, release less AVP, and more urine is produced.
[edit] Hemorrhaging and AVP release
- An experiment in rats shows that when blood loss exceeds 10% of the normal volume, AVP release increases rapidly and acts as a vasoconstrictor.
- AVP release is 10 times as much as standard water-maintaining AVP release levels.
- In this traumatic situation (large blood loss), AVP as a systemic vasoconstrictor in addition to acting as a water-retaining signal.
- Vasoconstriction increases blood pressure to the vital organs.
[edit] The body's response to excess hydration
- The body's response to hydration is similar to dehydration in that it functions through ADH modulation and is detected through osmoreceptors and baroreceptors.
- Upon excess hydration, the plasma osmolality decreases.
- Recall that water follows the osmolarity gradient; in the gut, the interstitum will have a much higher osmolality than the lumen, therefore water will flow into the body (the extracellular compartment).
- As the osmolality decreases, water will flow into the osmoreceptors of the hypothalmus and they will release less ADH, thus allowing the kidney to lose water.
- Upon excess hydration, the plasma volume increases.
- Recall that water will flow into the interstitium at the gut, then into the lymph and into the blood (plasma).
- Increased blood pressure will cause cardiovascular stretch receptors (in the atria) to signal for decreased ADH release from the hypothalamus / posterior pituitary.
Do these atrial stretch receptors signal via nervous firing? Do they signal to the hypothalamus, the post pit, or to the medulla?
[edit] Human response to drinking lots of water
- When humans drink lots of water, ADH can be completely shut off.
- Without any ADH expression, the collecting duct will not reabsorb any water but the PCT reabsorption will not change.
- The collecting duct becomes impermeable to water without ADH expression (that is, without aquaporin).
- Note that most of the excess water consumed was excreted within an hour of consumption.
- Also, recognize that water reabsorption is only moderated at the collecting duct which is only responsible for 10% of water reabsorption.
- However, because the kidney sees so much flow, we can retain or lose lots of water by just controlling that 10%.
- Recall, 10% of the kidney's filtrate volume is 18L / day of controllable volume.
[edit] Maintenance of effective arterial blood volume overrides
- Because AVP is important for regulating both plasma osmolality and blood volume, we wonder which one takes precedence.
- It seems that effective arterial blood volume (EABV) takes precedence over maintaining a certain plasma osmolarity.
- That is, when blood pressure is low, AVP will be released inspite of the fact that osmolality is decreased.
- This is important in disease states like congestive heart failure where the EABV (effective arterial blood volume) can appear low because the heart is not moving blood properly.
- While EABV (effective arterial blood volume) will be maintained via ADH, the osmolality of the plasma will decrease which can cause cellular swelling.
- Cellular swelling is especially bad in fixed volume areas like the cranium!
- That is, arterial blood volume will be restored at the expense of osmolality--even to the point of making the patient hyponatremic.
[edit] AVP increases water permeability of the collecting tubules
- Recall that AVP increases expression of the aquaporin proteins on the tubular cells of the collecting duct.
- AVP binds to the V2 receptor which uses a g-protein and adenylyl cyclase to increas cAMP.
- Thus AVP activates PKC and other proteins.
- Ultimately, AVP signaling through cAMP and PKC causes vesicles holding AQP (aquaporin) to be released such that aquaporin is integrated into the apical surface of the tubular cells.
- Increased AQP2 on the apical surface allows water to follow Na reabsorption.
- Also, AVP increases synthesis of the AQP2.
- Note, too, that AQP3 and AQP4 are always expressed on the basal surface of the principal cells.
[edit] Water permeability along the nephron
- Recall that water reabsorption occurs primarily in the PCT, the PST (proximal straight tubule), and the thin descending limb of Henle (tDLH).
- Also, water reabsorption can be increased via ADH's effect on the cortical collecting duct and the inner medullary collecting duct.
- In comparison, the proximal sections are nearly twice as water permeable as the distal regions even when the distal sections are AVP stimulated.
- started here on 03/25/11.
[edit] Dilution and concentration of urine
[edit] Why concentrate the urine?
- The kidneys concentrate the urine in order to save water for the body.
- About 600 mOsm of solute is secreted per day.
- If this were secreted at plasma concentrations, we would secrete 2 liters of water.
- When concentrated maximally, we can secrete this much solute in 0.5 liters of water.
- So we save 1.5 liters of water / day by concentrating the urine.
[edit] Hydration state calculations
- In considering the hydration state we care about how concentrated the urine is relative to the plasma and how much urine is being produced.
- The ratio of osmols in the urine to the plasma tells us whether more solute or more water is being lost:
- Uosm / Posm describes how concentrated the urine is relative to the plasma.
- The closer to infinite the ratio approaches, the more concentrated and the more water is conserved.
- The closer to zero the ratio approaches, the less concentrated and the more water is lost.
- Concentration of the urine is expressed in osmols: Uosm
- Concentration of the plasma is expressed in osmols, also: Posm
- When Uosm / Posm > 1, solute is being lost from the blood relative to water.
- When Uosm > Posm, we call the urine hyperosmotic; that is, water would rush out of a cell if placed in this urine.
- When Uosm / Posm = 1, solute and water are being lost from the blood at equivalent rates.
- In this case, we call the urine iso-osmotic to the plasma.
- When Uosm / Posm < 1, water is being lost from the blood relative to solute.
- When Uosm < Posm, we call the urine hypo-osmotic; that is, water would rush into a cell placed in this urine.
- Uosm / Posm describes how concentrated the urine is relative to the plasma.
- The next term of interest is Cosm, that is, the clearance of osmols.
- Given our ratio of urine and plasma concentrations (Uosm / Posm), we can develop a term that describes the net clearance of osmols by multiplying the ratio by the flow rate.
- Recall that V-dot represents the urine flow rate.
- Cosm = (Uosm / Posm) * V
- Cosm describes how much solute is being excreted as a function of flow rate (V), the concentration of the urine (Uosm) and the concentration of the plasma (Posm).
- In another sense, Cosm describes how much solute is being excreted as a function of flow rate (V) and water retention / loss (Uosm / Posm).
- Cosm is directly related to flow rate: as flow increases or decreases, solute excretion increases or decreases.
- Cosm can also be elevated by increasing the concentration of the urine or decreasing the concentration of the plasma.
- This makes sense because more concentrated urine contains relatively more solute than less concentrated urine, so it will clear solutes from the plasma more rapidly (that is, result in an elevated Cosm).
- Note that the concentration of the plasma does not change often.
- Given our ratio of urine and plasma concentrations (Uosm / Posm), we can develop a term that describes the net clearance of osmols by multiplying the ratio by the flow rate.
- A mis-named term called the "free water clearance" describes how much water is removed from the plasma to generate urine; that is, free water clearance can be used to determine if a patient is over- or under-hydrated.
- "Free water clearance" is denoted as CH20.
- "Free water clearance" is a misnomer because this does not really describe the "clearance" of water (as in the "clearance of aspirin").
- CH20 = V - Cosm = V - (V * (Uosm / Posm)) = V * (1 - (Uosm / Posm))
- Note that we are subtracting a portion of the flow from the flow itself.
- We know that the ratio of Uosm to Posm describes the clearance of osmols in terms of flow.
- So, to subtract the clearance of osmols from the flow gives us a term describing how much water was not cleared.
- That is, CH20 describes how much of the plasma was cleared of solute.
- When CH20 > 0, solute-free water is being removed from the plasma in order to dilute the urine.
- When CH20 < 0, solute-free water is being removed from the urine in order to concentrate the urine.
- When CH20 = 0, the urine is iso-osmotic relative to the plasma.
[edit] The long and short of Henle loops
- Species with a high proportion of long loops of henle (relative to short loops) can achieve greater urine concentration.
- Recall that long loops of henle drop deep into the medulla whereas short loops only superficially enter the medulla.
- 15% of human loops are long.
- The kangaroo rat rarely has to drink water because it has many long loops and can conserve so much of its water.
- The ability to produce osmotically concentrated urine is directly proportional to the length of the Henle loops.
- Desert dwellers have long loops, hydrated habitators have short loops.
[edit] Steep gradient of interstitial osmolarity
- The cortex has an interstitial osmolality of about 300 mOsm while the inner medulla osmolality is 1200 mOsm.
- This was determined from frozen rat sections of kidneys.
[edit] Kidney medulla contains two countercurrent mechanisms
- Recall that the whole point of the osmolarity gradient along the nephron is to allow water to flow down an osmolarity gradient across the collecting duct.
- So, the first thing to remember is that water only flows across the collecting duct when AVP is being expressed otherwise the collecting duct is impermeable to water.
- To understand the countercurrent mechanisms, remember that the kidney is trying to make the medulla highly filled with solute and very low on water (that is, the medulla should have a high osmolarity) so that water can flow from the filtrate (which is pretty concentrated at the collecting duct, into the medulla).
- Now, understand that the loop of henle establishes the osmolarity gradient and the vasa recta maintain the gradient.
- We say that the loop of henle is a countercurrent multiplier. That is, it puts solute into the medulla.
- We say that the vasa recta is a countercurrent exchanger. That is, it takes water out of the medulla.
[edit] Loop of Henle and countercurrent multiplication
- Recall that the whole point of the loop of Henle is to increase solute levels of the medulla.
- The loop achieves a high solute, low water distribution by alternating (along the descending and ascending loops) whether or not water or NaCl can escape the filtrate into the interstitium.
- As filtrate descends, water can escape into the interstitium but NaCl cannot follow.
- Note that this escape of water might seem to balance the pumping out of NaCl, but it will be removed from the medulla by the vasa recta.
- As filtrate ascends, NaCl is actively pumped into the interstitium but water cannot follow.
- As filtrate descends, water can escape into the interstitium but NaCl cannot follow.
- It is important to understand that NaCl is actively pumped; it is this burning of ATP that allows us to generate a gradient.
- Recall that without energy, nothing flows against its gradient.
- This active pumping is able to generate a gradient of 200 mOsm / kg between the descending and ascending filtrate; one can imagine that there is a limit to the gradient this pump can generate because of leaky channels, ATP turnover, et cetera.
- We call this a multiplication countercurrent because as the filtrate moves down and back up the tubule, the gradient is continually brought to a 200 mOsm difference such that the difference along the axis of the loop of Henle increases.
- In the loop of Henle, relatively more NaCl is pumped out (on the way up) than water is gained (on the way down) (that is the filtrate becomes more dilute), so the loop is often called the diluting segment of the nephron.
[edit] Vasa recta and countercurrent exchange
- Recall that the whole point of the vasa recta is to decrease the water levels of the medulla.
- Vasa recta are long, thin capillaries from the efferent arterioles of the juxtamedullary glomeruli.
- Recall that the loop of Henle let water enter the medulla through passive reabsorption in the descending loop.
- The vasa recta are the mechanism by which this water is removed from the medulla, resulting in a net gain of solute to the interstitial fluid (given all that is occurring at along the loop of Henle and the vasa recta).
- The vasa recta allow water to short circuit the path of the blood; that is, water will move from the descending branch of the vasa recta to the ascending.
- Water can short circuit the vasa recta loop because solutes (namely NaCl and urea) can also short circuit in the opposite direction.
- NaCl and urea that short circuit are said to be "cycling" because they can flow through the same stretch of vasa recta multiple times.
- Note that if the vasa recta blood flow rate is greatly increased, it can "wash out" the solutes from the medulla.
- That is, high blood flow rates can cause lots of solute reabsorption in from the medulla and decrease the osmolarity gradient along the cortical-medullary axis.
- This would result in a decreased ability to concentrate the urine and a decreased ability to shed water via urine.
So, if saline is given to increase the extracellular compartment and too much is given too fast, then the pt can't shed the excess via urine (because they can't concentrate urine), so then will edema and such result? Does edema only result after this point of flushing out the solute or can edema result before that?
[edit] Thermal models and countercurrent exchange
- Thermal models can help demonstrate how countercurrent exchange works.
- Imagine a source of heat at the bottom of the medulla:
- As filtrate flows down the descending limb to the source of heat it will heat up.
- As filtrate flows up the ascending limb (right next to the descending limb) it will heat the descending fluid next to it.
- Since the fluid now flowing down the descending limb is being heated by the ascending fluid and by the heat source itself (through conduction), there will be an increased temperature of that fluid when it hits the bottom.
- An equilibrium will be reached.
- There will be a nice heat gradient in the descending and ascending fluid as they flow along the cortical-medulla axis.
- And this gradient will generate a larger difference (larger gradient) than if one had just stuck a heat source at the bottom of the medulla and had no fluid flow.
[edit] The collecting duct and the interstitial osmolarity
- Now that the countercurrent mechanisms have set up this awesome osmolarity gradient, the collecting duct (which passes through the entire distance of the medulla) has an excellent opportunity to concentrate the urine.
- Recall that the collecting duct can only concentrate the urine via the countercurrent-generated osmolarity gradient when ADH is present.
- Without ADH, there is no aquaporin2 on the apical surface (lumen-facing surface) of the collecting duct principal cells and therefore no water flow from the filtrate to the interstitium.
- However, when ADH is present, AQ2 is present and water can flow down the osmolarity gradient (that is, the water concentration gradient) from the filtrate to the interstitium.
- When ADH is present, water flows from the filtrate, through AQ2, AQ3/4, into the interstitium, and then into the blood found in the vasa recta.
- When ADH is present, urine reaches the same concentration as the interstitial fluid at the papilla of the medulla.
- Urine concentration reaches about 1200 mOsm.
- There is decreased osmolarity gradient without ADH for several reasons:
- There is a small amount of water reabsorption even without ADH.
- There is a small amount of urea flow from filtrate to medulla even without ADH.
- There is increased medullary blood flow via vasa recta.
[edit] Nephron geography and reabsorption
- At the glomerulus, 100% of the initial filtrate is in the tubule.
- At the PCT, 30% of the initial filtrate remains in the tubule.
- At the DCT, 15-20% of the initial filtrate remains in the tubule.
- The amount of filtrate remaining in the collecting duct depends on the presence or absence of AVP:
- Without AVP, the collecting duct does not retain any water so 15-20% of the initial filtrate remains in the tubule (just like we saw in the DCT).
- With AVP, at the end of the collecting duct, 1% of the initial filtrate remains in the tubule.
[edit] Urea and the concentration of urine
- Recall that when ADH is present, the body is trying to reabsorb all the water it can.
- One effect of ADH on the collecting duct is to make the very medullary tip of the collecting duct permeable to urea (that is, urea can flow from the filtrate to the interstitium by chemical gradient).
- Urea movement from filtrate into medullary interstitial space is facilitated by specific urea transporters.
- As urea moves from the filtrate to the interstitium by chemical gradient it increases the osmolarity of the interstitium, thus drawing water with it.
- The water is then cleared from the medullary interstitium by the vasa recta.
- The urea ends up back in the filtrate and in the blood by way of vasa recta absorption and loop of Henle secretion.
- All this to say that urea is required for efficient urine concentration!
[edit] Urea and NaCl are the osmotic solutes
- So, urea in the medullary interstitium balances the urea in the collecting duct filtrate.
- Urea movement is facilitated but not active, so they can only balance one another.
- So, the NaCl that has been concentrated in the interstitium must provide enough osmotic force to counterbalance the osmotic pull of all the other solutes in the filtrate of the collecting duct.
- So the osmotic pull of urea and NaCl in the interstitium pull water from the collecting duct filtrate when everything is going well.
- Note that it is as of yet of unclear how NaCl is concentrated in the medullary interstitium given the fact that NaCl is passively reabsorbed at the ascending limb of the loop of Henle.
I thought that this occurred b/c of the active pumping of NaCl out of the filtrate into the interstitium at the ascending loop.
- The osmotic gradient and low blood flow make the intermedullary space a very hostile environment for cells.
[edit] Blood flow characteristics of the vasa recta
- Recall that the vasa recta is responsible for absorbing the water reabsorbed by the collecting duct upon ADH signaling.
- Recall that the descending loop of Henle lets water flow out of the filtrate.
- These two sources of water cause vasa recta outflow to be higher than vasa recta inflow.
- One can also describe this as a mass action balance: the osmolality of filtrate is decreasing along the way so the water has to go somewhere and it is going into the vasa recta; therefore the vasa recta's osmolality must be increasing (and thus flow must increase).
- Similarly, the filtrate flow will be decreasing as its osmolality decreases.
[edit] Factors that affect urine concentrating ability
- ADH: more ADH, more AQ2, more H20 reabsorption, more concentrated urine.
- NaCl at ascending limb of loop of Henle
**The more NaCl makes it to the ascending limb of the loop of Henle, ... Is NaCl the limiting factor in how much gradient can be generated between the ascending and descending loops by active NaCl secretion at the ascending loop?
- Reabsorption of NaCl by the ascending loop
**The more NaCl that is reabsorbed, the less osmolarity gradient there is and the less water is reabsorbed at the collecting duct.
- Delivery of fluid to medullary collecting ducts
**There is some threshold at which the collecting duct cannot reabsorb all the water passing through, even under ADH stimulation.
- Medulary blood flow
- If medullary blood flow is limited, water reabsorption from the interstitium by the vasa recta will be limited.
- Urea
- If urea is not available, less osmolarity gradient will be generated between the collecting duct filtrate and the medullary interstitium so less water will be reabsorbed.
- Length of Henle's loo
- The longer the loop, the greater the gradient can be formed along the cortical-medullary axis and the more water can be reabsorbed at the collecting duct.
- Aging
- As one ages, nephrons become less in number and less effective.
- Neurogenics diabetes insipidus
- Recall that neurogenic DI is caused by an under-expression of ADH.
- Decreased ADH means decreased AQ2, decreased water reabsorption, and dilute urine.
- We give exogenous ADH to treat neurogenic (central) diabetes insipidous.
- Nephrogenic diabetes insipidous
- Recall that nephrogenic DI is caused by a diminished response to ADH at the collecting duct.
- Most likely due to a disfunctional V2 receptor or AQ2 channel.
- We use thiazide diuretics and low Na diet to control volume and decrease GFR.
- Recall that thiazides inhibit the Na / Cl cotransporter at the DCT.
- Recall that nephrogenic DI is caused by a diminished response to ADH at the collecting duct.
- Primary polydipsia
- Primary polydipsia is the suppression of ADH by excessive fluid intake.
- This makes sense because if you drink lots of water, the body will decrease ADH to lose some of the water by urine.
- Both primary polydipsia (drinking lots of water) and nephrogenic diabetes insipidous will present as high urine production.
- How do you tell the difference between primary polydipsia and nephrogenic diabetes insipidous?
- One way is to take away water from the patient.
- A nephrogenic DI patient without water will continue to make dilute urine and will become dehydrated.
- A patient suffering from primary polydipsia will generate enough dilute urine to bring the body back to homeostatic water balance and then return to normal ADH expression and normal production of concentrated urine.
- stopped here on 03/25/11.
- started here on 03/28/11.
[edit] Sodium Balance
[edit] Objectives
- Compare the amounts of sodium which are filtered, reabsorbed, and excreted in a day.
- Indicate the percentages of filtered sodium that are reabsorbed in the proximal convoluted tubule, loop of Henle, distal convoluted tubule, and collecting duct.
- Explain how the following factors affect sodium excretion:
- glomerular filtration rate,
- mineralocorticoids (aldosterone),
- intrarenal physical forces,
- natriuretic hormones (atrial brain, and kidney natriuretic peptides; guanylin and uroguanylin; others),
- renal sympathetic nerves,
- changes in intrarenal distribution of blood flow, estrogens, osmotic diuretics, poorly reabsorbed anions, and diuretic drugs.
- Explain why the volume of the extracellular fluid is dependent on its sodium content.
- Discuss the input and output of sodium from the body.
- Describe the renal response to a change in dietary sodium intake.
- Explain how changes in effective arterial blood volume lead to changes in renal sodium excretion.
- Explain why renal salt and water retention is a key element in the development of generalized edema.
[edit] Kidneys are important to Na balance
- The kidneys filter lots of plasma yet retain nearly all of the Na; 99.6%.
- The kidneys even keep most of the K filtered, though K is sometimes secreted at the cortical collecting duct as part of a mechanism for retaining Na.
[edit] Na reabsorption along the nephron
- 70% of the filtered Na is reabsorbed in the proximal convoluted tubule.
- 90% of the filtered Na is reabsorbed by the end of the Loop of Henle.
- The most important Na regulation point is the collecting duct, though it does not reabsorb a huge percentage.
- There are four locations where diuretics take action: PT, LH, DT, and CD.
- Note that location of action determines effectiveness.
[edit] Factors that effect Na reabsorption
- There are a sooo many things that affect Na reabsorption:
- Glomerular filtration rate
- Mineralocorticoids (aldosterone)
- Intrarenal physical forces (Starling forces)
- Natriuretic hormones
- Renal sympathetic nerves
- Changes in intrarenal distribution of blood flow
- Estrogens
- Osmotic diuretics
- Poorly reabsorbable anions
- Diuretic drugs
[edit] Glomerular filtration rate
- GT (glomerulotubular) balance is this mechanism of employing the post-PCT nephron to reabsorb Na so that large losses don't occur simply because of the glomerular filtration rate.
- We know that the proximal tubule reabsorbs 2/3 of the Na in the filtrate regardless of GFR.
- Therefore, if GFR goes up and PCT Na reabsorption does not change, an increase in GFR causes an increase in Na remaining in the filtrate post-PCT.
- That is, if the PCT always reabsorbs 2/3, then as the flux increases, the amount of Na still in the post-PCT filtrate will increase, too.
- Be careful because Na excretion and Na reabsorption are affected exactly opposite as GFR changes.
- Understand that this means that as GFR goes up, the volume of Na that does not get reabsorbed in the PCT increases proportionally.
- This increased amount means that the down-stream parts of the nephron have to reabsorb more or there will be a larger increase in Na.
- The reabsorption increase capacity of the post-PCT nephron picks up the slack to keep Na loss from occurring.
- So we say that GT balance (glomerulotubular balance) is a way to maintain a constant reabsorption of Na even when PCT reabsorption doesn't change.
- Recall that we can calculate the filtered Na load = GFR * [Plasma Na]
- One must be able to do this calculation.
[edit] Mineralocorticoids (aldosterone)
- Recall that aldosterone (from the glomerulosa of the adrenal gland) causes principal cells of the collecting duct to put ENaC on their apical membrane and Na / K ATPase on the basolateral membrane thus increasing water reabsorption.
- Recall that ENaC is a passive channel and it is the movement of Na by Na/K ATPase that drives increased Na reabsorption.
- Note that K is pumped into the cell by Na / K ATPase and then is drawn into the filtrate via K channels by osmotic force; this is the location / mechanism of K-wasting.
- Secretion of aldosterone is primarily controlled by the renin-angiotensin pathway.
- We know that a baseline level of aldosterone is always secreted because pts with adrenalectomy only reabsorb 98% of their filtered Na instead of 99.6%.
- Addison's Disease is a disease of adrenal insufficiency; that is Addison's disease results from too little aldosterone production.
- If there is too little aldosterone, then too little AQ2 is put on the collecting duct cells and too little water is reabsorbed.
- So we expect Addison's disease to lead to: nausea, vomiting, low blood pressure that falls further when standing, causing dizziness or fainting (hypovolemia), a craving for salty foods due to salt loss
Why does nausea and vomiting result from Na wasting?
- Aldosterone escape:
- Aldosterone escape is a phenomenon in which the kidney--under high doses of mineralocorticoids like aldosterone--stops responding to the mineralocorticoid as expected (that is, the kidney stops retaining water even in the presence of mineralocorticoid signaling).
- Note that DOCA is a potent mineralocorticoid.
- Aldosterone breakthrough:
- Aldosterone breakthrough is a phenomenon in which the adrenals--under high doses of ACE-inhibitors--re-establishes normal aldosterone levels.
- That is, ACE-inhibitors are given (let say, to reduce blood volume in an hypertensive pt) so renin levels are high, angiotensin II levels are low and aldosterone levels are initially low (which is achieving our purpose of less water reabsoprtion and less extracellular volume) and then aldosterone levels return to pre-treatment levels.
[edit] Intrarenal physical forces (Starling forces)
- Recall that Starling forces include hydrostatics and colloid (represented by pi) osmotic pressures and determine flow in and out of capillaries.
- These forces affect reabsorption at the PCT, also.
- Saline treatment:
- Adding saline will increase the extracellular volume and therefore increase the capillary hydrostatic pressure (more volume in the same system means more pressure) and decrease the blood osmotic pressure drawing water into the blood (same amount of protein in the extracellular compartment but more water and Na).
- Note that this means less force drawing Na and water into the blood.
- Note that treating with DOCA is an endogenous way to add saline because DOCA will cause increased Na reabsorption (via ENaC and Na / K ATPase) and water will follow.
- Adding saline will increase the extracellular volume and therefore increase the capillary hydrostatic pressure (more volume in the same system means more pressure) and decrease the blood osmotic pressure drawing water into the blood (same amount of protein in the extracellular compartment but more water and Na).
- In response to Saline / DOCA treatment (pressure natriuresis):
- Capillary hydrostatic pressure has increased and blood osmotic pressure has decreased so there is less force moving Na and water into the blood.
- So the renal response to saline / DOCA is a decrease in reabsorption of Na, widening of tight junctions and subsequent back-leak of Na into filtrate and thus increased Na excretion.
- This phenomenon of backlead because of increased hydrostatic pressure and decreased osmotic pressure is called pressure natriuresis.
[edit] Natriuretic hormones
- Atrial natriuretic peptide is released by endocrine cells of the cardiac atria in response to stretch receptors.
- That is, when extracellular fluid levels are high and atria are "overfilled" during the cardiac cycle, atrial natriuretic peptide is released.
- ANP (atrial natriuretic peptide) acts on collecting duct and afferent arteriole.
- ANP on the collecting duct:
- ANP acts on the collecting duct to decrease ENaC on the apical membrane and decrease Na / K ATPase on the basolateral membrane. That is ANP has the exact opposite effect of aldosterone.
- Recall that less ENaC and less Na / K ATPase will cause less reabsorption of Na and therefore less water reabsorption and less water in the extracellular compartment (to fix the atrial over-filling problem).
- ANP on the afferent arteriole:
- ANP dilates the afferent arteriole which increases GFR.
- Recall that increased GFR means increased filtrate flow rate and decreased Na reabsorption (and therefore less water reabsorption and less volume in the extracellular compartment).
- ANP-like peptides from other organs:
- The kidney has an ANP-like protein called urodilatin.
- The brain has B-type ANP.
- B-type ANP has actually been used clinically for patients with congestive heart failure.
[edit] Renal sympathetic nerves
- Sympathetic innervation is used to decrease renal blood flow and GFR in traumatic situations.
- Recall that decreased GFR will mean slower filtrate movement, more Na reabsorption, and increased water retention which is good when your leg has been chopped off.
Why does reduced RBF (renal blood flow) cause increased Na retention?
[edit] Changes in intrarenal distribution of blood flow
- Recall that there are two types of glomeruli based on their location and how deep their vasa recta / LoH run: cortical glomeruli and juxtamedullary glomeruli.
- Recall that the juxtamedullary glomeruli have deeper-running vasa recta / LoH and can therefore generate more concentrated filtreate (that is, reabsorb more of the Na / water from filtrate).
- Shifting blood flow from the cortical glomeruli to the juxtamedullary glomeruli can cause increased Na reabsorption.
- This is not a fully accepted reality among renal physiologists.
[edit] Estrogens
- We know that Na and water are retained at higher levels when estrogen levels are elevated (via ovarian cycle or exogenous delivery).
- We suspect that estrogen may have a direct effect on tubular reabsorption.
[edit] Osmotic diuretics
- Recall "osmotic diuresis" means "peeing because of osmotic pull of water toward filtrate".
- Osmotic diuresis can be induced by exogenously delivering a large amount of a small molecule (like mannitol) that is excreted via the urine.
- The presence of the mannitol in the filtrate increases the osmotic force of the filtrate and inhibits Na reabsorption.
- Note that Na reabsorption still occurs but only until the principal cell's intracellular Na concentration is high enough to shut off Na reabsorption from the filtrate.
- Osmotic diuresis is possible because the distal nephron has limited capacity to move Na against an osmotic gradient.
[edit] Poorly reabsorbable anions
- As a positively charged ion, Na+ may remain in the filtrate (that is, not be reabsorbed) when there are many negatively charged ions that command a the presence of a balancing positively-charged ion.
- Examples of negatively charged ions that inhibit Na reabsorption include sulfate, phosphate, and ketones.
[edit] Diuretic drugs
- Note that all diuretics work by blocking Na reabsorption, but each works at a different location on the nephron.
- Osmotic diuretics work at the proximal tubule by putting so many non-reabsorbed small molecules into the filtrate that there is much osmotic pressure keeping water from being reabsorbed.
- Furosemides function at the thick ascending limb of the Loop of Henle by inhibiting the Na/Cl/K triple-co-transporter.
- Note that furosemides have a high ceiling meaning one can increase the dosage with a linear effect over a long range of dosages.
- Note that when furosemides inhibit reabsorption of Na they also inhibit reabsorption of K, so it is considered a potassium-wasting diuretic.
- Amiloride acts at the collecting duct by inhibiting ENaC.
- Recall that ENaC is able to move Na from filtrate into the cell because there is a basolateral Na / K ATPase keeping the intracellular concentration of Na low; consequently as Na in the filtrate goes up, Na / K ATPase activity goes up to keep intracellular Na at a low level, intracellular K goes up (Na and K of Na / K ATPase go in opposite directions), and K is lost to the filtrate.
- We call this "the washing away of K".
- Amiloride is considered a potassium sparing diuretic because when ENaC is inhibited, Na / K ATPase doesn't pump K into the cell and K is not then lost to the filtrate.
- Recall that ENaC is able to move Na from filtrate into the cell because there is a basolateral Na / K ATPase keeping the intracellular concentration of Na low; consequently as Na in the filtrate goes up, Na / K ATPase activity goes up to keep intracellular Na at a low level, intracellular K goes up (Na and K of Na / K ATPase go in opposite directions), and K is lost to the filtrate.
Do aldosterone levels rise when amiloride is given? Does one have to compensate with more amiloride?
- Thiazides act at the DCT by inhibiting the NaCl cotransporter.
- Note that thiazides have a low ceiling meaning one can increase the dosage but will hit a maximum effect quickly.
- Note that when furosemides inhibit reabsorption of Na at the DCT, more Na gets to the collecting duct where ENaC and Na / K ATPase will function at high levels to maintain a low intracellular Na and thus K will be moved into the fluid; thus thiazide's decrease of Na at the DCT causes an increase of K loss at the collecting duct.
- Spironolactone works at the collecting duct by competing with aldosterone for aldosterone receptors (which would signal for ENaC placement on the apical membrane of principal cells).
- Note that spironolactone is considered a potassium-sparing diuretic for the same reason as amiloride--inhibiting the movement of Na by ENaC means Na / K ATPase doesn't pump as much K into the principal cell to be lost to the filtrate.
For my logic to work regarding K loss at the CD by way of ENaC / Na-K ATPase, it must be the case that Na-K ATPase's function is regulated by intracellular Na levels. Is this the case? I know it is regulated by the cAMP levels, so as long as those are kept constant, then this seems to be correct logic.

[edit] Na is the main extracellular ion
- To demonstrate the importance of sodium (Na) in maintaining osmotic homeostasis regard the summation of the three major osmolytes in the ECF (Na, Glucose, and BUN):
- Recall that plasma osmolarity (osmolarityplasma) is maintained at about 285 mosm / kg.
- Osmolarityplasma = 2 * [Na] + 1/18 * [glucose] + 1/3 * [BUN]
- Osmolarityplasma ~= 2 * [140] + 1/18 * [100] + 1/3 * [10]
- Osmolarityplasma ~= 280 + 18 + 3
- Osmolarityplasma ~= 301 mOsm (of the normal 285; so not exact but shows that [Na] is super important)
- The volume of the extracellular compartment is directly proportional to the amount (that is, the number of molecules) of Na.
- That is, concentration of Na is regulated to remain constant: concentration of Na = amount (number of molecules) / volume.
- To hold concentration constant, the volume is changed to meet the amount.
[edit] The kidney maintains Na balance
- The kidney is responsible for 95% of the Na loss and gain (that is, 95% of the Na control).
- Recall that Na input includes only the diet and loss includes feces, urine, and sweat.
- Recall that Na can move within compartments of the body and that these movements are regulated: extracellular fluid, bone, intracellular compartments.
- Therefore, when the kidneys keep or rid too much Na, the ECF compartment can change drastically.
- The response of the kidney to changes in dietary sodium (the largest variable in a healthy pt's Na regulation) lags behind the change in intake.
- When dietary Na intake goes up and the kidney has not yet responded, a patient is in positive Na balance.
- Positive Na balance is accompanied with weight gain through water retention.
- Eventually, the kidney will decrease Na reabsorption and allow Na and water to be excreted via urine to reach normal ECF levels of Na and therefore normal ECF volume.
- When dietary Na decreases and the kidney has not yet responded, a patient is in negative Na balance.
- Negative Na balance is accompanied with weight loss through water loss.
- Eventually, the kidney will increase Na reabsorption and retain Na and water from the filtrate to reach normal ECF levels of Na and therefore normal ECF volume.
- If a pt gains 280 mEq of Na in a period of positive Na balance, how much weight would be gained?
- Recall that concentration of Na is held constant so concentration = amount / volume must remain constant.
- Volume will be increased proportionally to the increase in Na amount.
- Recall that a normal ECF Na concentration is 140 mEq / liter and a normal ECF volume is 15 liters.
- That is a normal total ECF Na amount is 2100 mEq / 15 liters.
- Therefore, as Na amount goes up by 280 mEq (2/15ths of the total), volume must also go up by 2/15ths (2 liters).
- Each liter of water weighs 1 kilogram (and each kilogram weighs ~2 lbs); 2 kg (~4 lbs) are gained.
- You could pose the question as "how much water should you add to your diet to make your input isoosmotic?".
[edit] Regulation of the effective arterial blood volume (EABV)
- The effective arterial blood volume is considered a synonym for the ECF.
- EABV (effective arterial blood volume) is regulated by negative feedback systems of the kidney and the cardiovascular system.
- Renal juxtaglomeruli apparati are the sensor for the negative feedback mechanisms of the kidney.
- These will activate renin-angiotensin-(aldosterone-ENaC and vasoconstriction) mechanisms.
- Cardiovascular stretch receptors are the sensor for the negative feedback mechanisms of the cardiovascular system.
- These will activate ANP-(ENaC-reduction and vasodilation) mechanisms.
- Note that sweating doesn't cause hyponatremia; sweating causes hypovolemia because 'sweat is hypoosmotic relative to blood (that is, there is more water per solute in salt than in blood).
- stopped here on 03/28/11.
- started here on 03/29/11.
[edit] K, Pi, Ca, and Mg Balance
[edit] Objectives
- Discuss the amount of potassium in the body and its distribution.
- Define hypokalemia and hyperkalemia.
- Indicate how the following factors affect the distribution of potassium between intracellular and extracellular fluids: Na+/K+-ATPase activity, plasma pH, insulin, epinephrine, plasma osmolality, tissue trauma, infection, and hemolysis.
- Identify the sites of K+ reabsorption and secretion along the nephron and collecting duct system.
- Draw a cell model for K+ secretion by a cortical collecting duct principal cell.
- Explain how the following affect potassium excretion: dietary potassium intake, mineralocorticoids (aldosterone), acid-base disturbances, excretion of poorly reabsorbed anions, and sodium excretion (tubule fluid flow rate).
- Explain how the kidneys keep maintain phosphate and calcium balance.
- Indicate the important sites of reabsorption of phosphate and calcium along the nephron.
- State the effects of parathyroid hormone (PTH) on tubular reabsorption of phosphate, tubular reabsorption of calcium, renal synthesis of 1,25-dihydroxy vitamin D3, and bone resorption.
- Identify the major site of magnesium reabsorption along the nephron.
[edit] Renal potassium regulation
- Note that potassium input is from diet and output is via feces and urine.
- Note that potassium movement within compartments is regulated: ECF, intercellular fluid, bone / connective tissue, trancellular fluid (CSF, etc.).
[edit] The importance of Potassium
- Potassium is important for several physiological reasons.
- Potassium affects the volume of cell as it is the primary osmolyte that gets moved across cellular membranes.
- Potassium affects excitability as it is the dominant ion in determining the transmembrane potential.
- Potassium affects pH (acid-base) balance.
- Potassium affects cell metabolism as it is involved in tissue growth and repair.
- In a healthy male, 89% of the body's K is intracellular, 8% is in bone / cartilage, 2% is in the ECF, and 1% is transcellular (CSF, etc.).
- A normal plasma K is 3.5-5 mEq / L; anything above or below is hyper- / hyper-kalemia.
- Note that hyperkalemia reaching 7 mEq / L is dangerous and 10-12 mEq / L of potassium is usually fatal via cardiac arrhythmia / arrest.
[edit] Movement of K between ICF and ECF
- There are many reasons K moves out of cells: low ECF pH, digitalis, O2 lack, hyperosmolality, hemolysis, infection, ischemia, and trauma.
What's the difference between O2 lack and ischemia?
- There are several reasons K moves in to cells: elevated ECF pH, insulin, epinephrine.
- Clinically, hyperkalemia can be treated with sodium-bicarbonate (IV) which will push K into cells by alkalinizing the ECF (that is, elevating the ECF pH).
- Hyperkalemia can also be treated with insulin + glucose (IV) as increased insulin drives K in to cells.
[edit] Kidneys filter, reabsorb, secrete, and excrete K
- Recall that we filter 180 L of plasma each day.
- Potassium (K) is normally maintained at 4 mEq / L.
- For comparison, recall that Na is maintained at 140 mEq / L.
- About 90 mEq of potassium are excreted each day (about 12.5% of the potassium filtered).
- Recall that only 0.9% of the Na filtered each day is excreted.
- Potassium (K) is normally maintained at 4 mEq / L.
- The kidneys excrete 90% of our daily intake of potassium.
[edit] K reabsorption within the nephron
- As with Na, most of the secretion / regulation of K occurs at the collecting duct.
- Just as only about 10-15% of the water of filtrate makes it to the collecting duct, only about 5% of the K of filtrate makes it to the collecting duct.
- 70% of filtered K is reabsorbed in the PCT (like Na).
- 25% of filtered K is reabsorbed in the LoH.
- Recall that the descending branch is permeable to water and solute in both directions.
- Recall that the ascending branch is impermeable to water and actively reabsorbs Na (actively pumps Na out of the filtrate).
- When plasma K levels are low, the collecting duct and continue reabsorption until only 1% of filtered K remains in the filtrate.
- When plasma K levels are high, the collecting duct can secrete K such that the filtrate contains 150% as much K as was filtered.
- Recall that normal physiological state results in 15% of the filtered load being excreted in the urine.
[edit] K secretion at the collecting duct
- The main site for regulated K secretion is the principal cells of the collecting duct.
- Recall that the principal cells of the collecting duct use ENaC and Na-K ATPase to regulate Na reabsorption.
- In a similar manner, principal cells use their basolateral Na-K ATPase to drive up the intracellular K concentration and their apical K channels to allow the K to flow into the filtrate via electrochemical gradient.
- So, just as the Na-K ATPase moves Na and K in opposite directions, the channels on the membrane allow them to flow opposite directions (out of and into the filtrate, respectively).
- So, principal cells respond to elevated K via their basolateral Na-K ATPase and apical K channel.

[edit] K reabsorption at the collecting duct
- The main site for regulated K reabsorption is the type A intercalated cells of the collecting duct.
- Recall that type A and type B intercalated cells of the collecting duct regulate blood pH by secreting H+ and reabsorbing HCO3- (type A cells) or secreting HCO3- and reabsorbin H+ (type B cells).
- Recall that intercalated cells (both types) generate H+ and HCO3- via the protein AE1.
- Recall, too, that intercalated cells secrete / reabsorb H+ by way of an apical H+ / K+ ATPase: that is, they burn ATP to exchange H+ / K+ at the apical membrane.
- Type A intercalated cells secrete H+ to balance an acidotic plasma and do so by reabsorbing K+ from the filtrate.
- Therefore type A intercalated cells are the primary location of K reabsorption in the nephron.
- type A intercalated cells respond to aldosterone-induced K loss:
- When mineralocorticoids are found in excess (think aldosteronism), Na is heavily reabsorbed at the collecting duct (think ENaC and Na-K ATPase) at the expense of K.
- Therefore type A intercalated cells attempt to compensate for the aldosterone-triggered K loss by secreting acid in exchange for K.
- This is the mechanism for renal alkalosis compensation.
- Recall that the body can compensate elevated pH (alkalosis) via the lungs (hypoventilation, breath off less CO2) or the kidneys (secrete H+ into the filtrate).
[edit] Factors that increase K excretion
- There are several factors that increase K excretion; some are pro-reabsorption effects and some are anti-reabsorption effects.
[edit] Increased dietary K intake increases K excretion
- As dietary K increases, plasma K will increase.
- As plasma K increases both the adrenal glomerulosa cells and the principal cells of the collecting duct respond.
- Glomerulosa cells of the adrenal glands increase production of mineralocorticoids in response to elevated plasma K.
- Increased amounts of mineralocorticoids leads to increased exchange of Na for K at the collecting duct and therefore increased K excretion.
*Principal cells of the collecting duct will How do principal cells respond to elevated plasma K levels?
[edit] Increased mineralocorticoids increases K excretion
- Recall that mineralocorticoids generally have a delay of hours before their response because they are steroids that bind intracellular receptors that act as gene expression regulators.
- As mineralocorticoids increase (think aldosterone), more Na is exchanged for K at the collecting duct (ENaC / Na-K ATPase).
- Exchange is increased because the number relevant surface proteins is increased (ENaC, Na-K ATPase, and K channels) and the production of ATP is increased.
- As more K is pumped into the principal cell more will flow into the filtrate by way of electrochemical gradient and K channels
[edit] Increased blood pH increases K excretion
- As the blood pH rises, the type A intercalated cells (think "aaaaah! too much aaaaacid!") respond by pumping H+ out of the blood into the filtrate in exchange for K+.
- As H+ is pumped into the filtrate (from the type A intercalated cell cytoplasm), K+ is brought into the cytoplasm and then moves into the blood via electrochemical gradient and K channels.
- Note that in acute acidosis the converse occurs: high levels of H+ in the ECF leads type B cells secreting H+ in exchange for K+ from the filtrate and thus K+ excretion is decreased.
[edit] Increased concentration of poorly absorbed anions increases K excretion
- Recall that an electrochemical gradient occurs over the apical membrane of the epithelial cells that line the nephron tract and that the gradient must be (at least somewhat) charge balanced.
- That is, the electrochemical gradient over the membrane cannot be super large.
- Therefore, if many negatively charged ions exist in the filtrate there must be some positively charged ions present also.
- As the concentration of anions increases, the movement of K out of principal cells increases (that is, K excretion increases).
[edit] Increased Na+ excretion increases K excretion
- Recall that elevated Na excretion means there is increased flow of filtrate (water follows Na, so if the amount of Na is elevated so is the volume of filtrate).
- When filtrate volume increases, so does the flow rate (more volume through the same area).
- As the flow rate increases there is an increase in electrochemical pull of K out of the principal cells and into the filtrate.
- Furthermore, for Na excretion to increase, the collecting duct principal cells must not reabsorb all the Na--but, all the apparatus for reabsorbing Na (think ENaC, Na-K ATPase) that does exist on the cell membranes will probably be working at maximum capacity (even if there is very little of it because there is very little aldosterone signaling) so K will be sacrificed for the Na (via the aforementioned Na-K ATPase).
[edit] Na deprivation does not lead to K excretion
- Now that we know that K is sacrificed in order to reabsorb Na, we might think that a pt deprived of Na would end up getting rid of lots of K (that is, filtrate would be low in Na, jg apparatus detects, signals granular cells to release renin, renin generates angiotensin, angiotensin stimulates the glomerulosa layer of the adrenal cortex, aldosterone released, ENaC and Na-K ATPase added to membranes of principal cells and K would be pumped into the filtrate in order to reabsorb all the Na out of the filtrate possible).
- However, there is a second mechanism that balances this this reaction and yields no extra loss in Na.
- (In the case of Na deprivation) filtrate Na would be low, jg apparatus detects, signals renin release, angiotensins are at high levels, both the afferent and efferent arterioles constrict, GFR decreases, Na reabsorption in the PCT increases, flux through collecting duct decreases, and decreased electrochemical pull of K out of the principal cells occurs.
I thought the PCT's reabsorption of Na was constant? (Na reabsorption lecture)
- In short: the aldosterone response increases K excretion but the GFR (decrease) response decreases K excretion.
[edit] Potassium excretion deficiency
- Potassium excretion is deficient in Addison's disease and renal failure.
- Recall that aldosterone tells the collecting duct to express ENaC and Na-K ATPase.
- Recall that as Na is reabsorbed via ENaC, it is driven by the Na-K ATPase-generated Na gradient and therefore Na is retained at the expense of K lost to the filtrate.
- Recall that Addison's disease is a deficiency in aldosterone production.
- In renal failure, there is deficient response to aldosterone.
- Poor aldosterone production / response means poor expression of ENaC / Na-K ATPase and poor reabsorption of Na / loss of K.
- Chronic renal failure is defined as GFR < 20 ml / min.
- Note that deficiency in excretion can lead to hyperkalemia.
[edit] Potassium excretion excess
- Potassium excretion is elevated by hyperaldosteronism and (most) diuretics:
- When aldosterone is elevated (as in hyperaldosteronism), lots of ENaC is put on the apical membrane of principal cells of the collecting duct and lots of Na-K ATPase activity causes lots of K moved into the cell and thus loss of K into the filtrate via electrochemical pull.
- Similarly, most diuretics decrease Na reabsorption upstream of the collecting duct and thus there is more Na in the filtrate to be reabsorbed by ENaC / Na-K ATPase at the collecting duct and thus lots of K movement into the principal cells and subsequently lots of K loss to the filtrate due to electrochemical gradient.
- Note that excessive potassium excretion will lead to hypokalemia.
[edit] Renal regulation of Pi
[edit] The importance of Pi
- Pi is an important buffer in the plasma and urine; recall that Pi usually has one or two H bound which it can release to buffer bases.
- A normal plasma Pi level is 1 mM.
- There is usually a 4:1 ratio of HPO4 to H2PO4.
- Most of the Pi in the body is stored in the bone, but some of the bone is remodeled daily such that Pi is released and added back allow for an exchange of bone Pi for dietary Pi.
- Usually about 2/3 of our dietary Pi is absorbed and an equal amount is excreted at the kidney.
[edit] Kidneys filter, reabsorb, and excrete Pi
- Phosphate is easily filtered by the glomerulus.
- Most of the filtered Pi is reabsorbed at the proximal tubule (and very little is reabsorbed beyond the proximal tubule).
- There is a Na-Pi cotransporter on the apical (brush border) membrane of the proximal tubule cells.
- Recall that Tm is the maximum rate at which a transporter can move molecules across a membrane.
- The amount of filtered Pi often exceeds the Na-Pi cotransporter's Tm and thus Pi is generally lost to the filtrate.
- Recall that this isn't all bad, though, because Pi is a nice buffer molecule for the urine.
- PTH is the primary regulating molecule / mechanism for Pi
- Recall that PTH is released by the parathyroid to increase Ca levels.
- PTH causes rapid endocytosis of the Na-Pi cotransporter at the proximal tubule and thus increased Pi loss.
[edit] Renal failure leads to bone disease, precipitates, and renal transplant
- During renal failure, there is poor PTH response by the proximal tubule reabsorption cells and therefore an increase in plasma Pi called hyperphosphatemia.
- Recall that a decrease in PTH response means less Na-Pi transporter removed from the surface; that is, decreased PTH response leads to increased plasma Pi levels.
- Renal failure also leads to decreased tubular mass (cells are dying).
- Both increased plasma Pi and decreased VitD activation lead to less VitD activation at the kidney.
- Recall that VitD is made at the skin and activated at the liver and kidneys.
- Decreased VitD activation leads to decreased Ca reabsorption at the gut.
- Recall that VitD is a necessary transcription factor for some protein that helps transport Ca across the enteric cell cytoplasm.
- Decreased Ca reabsorption at the gut means less Plasma Ca.
- Less plasma calcium leads to more PTH release.
- Elevated PTH leads to Ca and Pi release at the bone.
- Ca and Pi release at the bone leads to bone loss, Ca-Pi precipitation, and brittle blood vessels.
Q: In "renal failure" how do we know what fails? For example we say "renal failure leads to hyperphosphatemia" but the etiology of all these failures in regulation are not explained. It could be the PTH signaling or the apical membrane protein that moves Pi or.... A: We define renal failure (at least chronic) as a GFR < 20 (mL / hour). Therefore, "failure" or deficiencies in regulation occur because there is such a low volume of plasma being filtered that the regulatory mechanisms of the renal tubule cells (think acid secretion, Na reabsorption, etc.) cannot be effective.
[edit] Renal regulation of Ca
- Normally, only 20% of dietary Ca is absorbed.
- This can be increased by VitD.
- Like Pi, most of the body's Ca is in the bone but some of the bone is remodeled each day providing an exchange of Ca between compartments.
- Also like Pi, in a stable situation the amount of Ca absorbed by the gut is matched by the amount secreted by the kidney.
- A normal plasma Ca is about 2.5 mM.
- Recall that normal Pi plasma level was 1 mM.
- Unlike Pi, about 50% of the plasma Ca is bound to plasma protein and therefore not filtered.
[edit] The importance of Ca
- Ca is a very tightly regulated plasma ion.
- Ca is important for cellular depolarization and as a cofactor for many cellular reactions.
[edit] Kidneys filter, reabsorb, and excrete Ca
- Recall that 50% of the plasma Ca is bound to plasma protein and therefore not filtered.
- Ca reabsorption in the nephron uses an apical Ca channel and basolateral active transport.
- Filtered Ca is reabsorbed primarily by the PCT (60%) and the LoH (30%).
- Regulated reabsorption of Ca occurs in the ascending limb, the DCT and the connecting tubule.
- Here, PTH increases the reabsorption of Ca (mechanism not given).
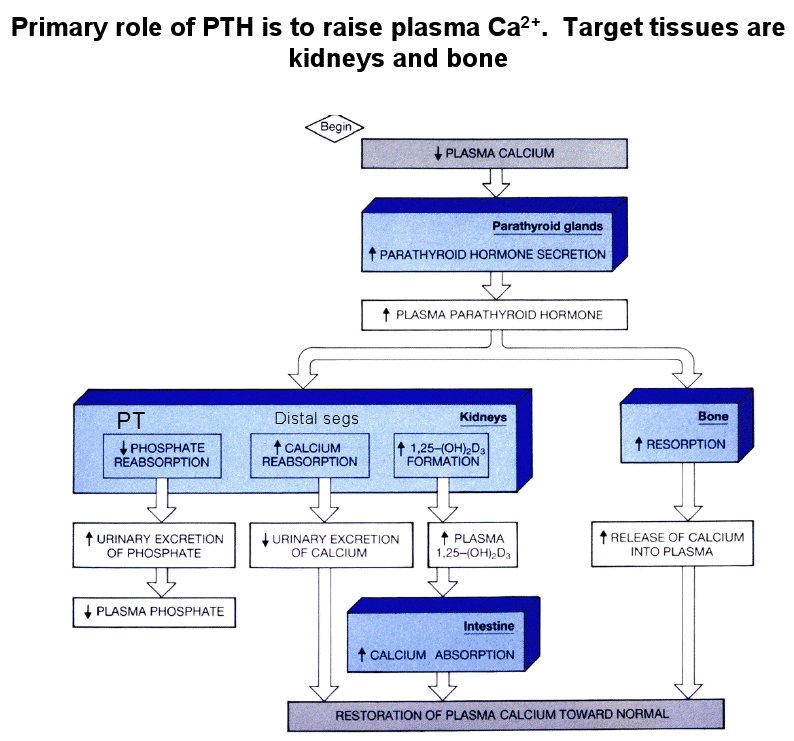
[edit] Renal regulation of Mg
- 99% of Mg is in bone and cells.
- Mg is neglected but second only to K in abundance.
- A normal plasma Mg is 0.8-1.0 mM
- Recall that a normal plasma Ca is 2.5 mM.
- Recall that a normal plasma Pi is 1.0 mM.
- 30% of plasma Mg is bound to protein.
[edit] The importance of Mg
[edit] Kidneys filter, reabsorb, secrete, and excrete Mg
- Recall that 30% of plasma Mg is protein-bound and therefore not filtered.
- The kidney reabsorbs 90% of the filtered Mg.
- Reabsorption of Mg occurs primarily in the loop of Henle (60% of the renal reabsoprtion)
- Mg reabsorption occurs by paracellular movement, driven by the positive potential of the filtrate that wants to push positive ions like Mg2+ out.
- Recall that "paracellular movement" means the Mg moves from filtrate to interstitial fluid by going around the junctions of the cells.
- Similar movement is achieved by other positive ions, too: Na+, K+, Ca++, and NH4+.
- stopped here on 03/29/11.
[edit] Acute kidney injury
[edit] Definition
- We have renamed acute renal failure (AFR) to acute kidney injury (AKI).
- We renamed it because of an historic inability to sufficiently define ARF.
- Now we define acute kidney injury (AKI) as "a functional or structural abnormality of the kidney as determined by blood, urine, or tissue tests or imagine studies that manifests with 48 hours".
- We use diagnostics like serum creatinine (normally cleared by the kidney) and urine production to determine the occurrance of AKI (acute kidney injury).
- Serum creatinine increase by 50% or over 0.3 mg / dL is considered diagnostic for AKI.
- Reduced urine production resulting in less than 0.5 ml / kg / hr for 6 hours is diagnostic for AKI.
- Oliguria: "production of an abnormally small amount of urine" per wornet
[edit] Epidemiology
- The incidence of AKI (acute kidney injury) depends on the setting:
- hospital population: 5-7%
- ICU: 15-40%
- community: 1%
- The mortality of AKI (acute kidney injury) ranges from 36-86% and depends on:
- setting ICU > hospital > community
- age
- illness acuity
- severity of injury
- Elevated serum creatinine levels, even with only mild increases, can result in large increases in mortality.
[edit] Pathophysiology
- There are many ways that the kidney can be injured:
- decreased perfusion
- toxins
- ischemic or obstructive injury to the tubule
- inflammation and / or edema of the tubulointerstitium,
- primary glomerular disease
- We divide the pathophysiology into three, broad, anatomical divisions: prerenal, intrinsic / intrarenal, and post-renal.
- Prerenal makes up 40-70% of AKI pathophys
- Intrinsic makes up 25-40% of AKI pathophys
- Postrenal makes up 5-10% of AKI pathophys
- Intrinsic pathophysiology will be the focus of our inquiries and includes several types of injury:
- acute glomerulonephritis
- acute interstitial nephritis
- tubular cell injury
- Ischemia and inflammation (sepsis, surgery, hypoperfusion)
- Toxins (direct or indirect)
[edit] Prerenal AKI
- Azotemia: "Azotemia is a medical condition characterized by abnormally high levels of nitrogen-containing compounds, such as urea, creatinine, various body waste compounds, and other nitrogen-rich compounds in the blood." per wikipedia
- Azotemia is the most common etiology of prerenal AKI.
- Azotemia can occur when the extracellular compartment undergoes a severe decrease in volume.
- We categorize azotemia into volume-responsive or not depending on whether expanding the extracellular compartment resolves the azotemia.
- For example, adding saline will expand the extracellular compartment.
- Another major cause of prerenal AKI is medications that work upstream of renal function: angiotensin converting enzyme inhibitors (ACEI’s), angiotensin receptor blockers (ARB’s) and NSAIDs (reduce glomerular
capillary perfusion by reducing prostaglandins such that vasodilation is reduced).
- Prerenal AKI timeline:
- autoregulatory mechanisms of the kidney attempt to maintain blood flow and GFR (think myogenic and tubuloglomerular feedback),
- Recall that these serve to change the blood flow in the afferent and efferent glomerular arterioles
- renal hypoperfusion triggers the hormonal response (think renin, aldosterone, and AVP) which mostly maintains the GFR
- without interventsion, the pt progressive to significant tubular cell injury due to ischemia (which is then intrinsic AKI).
- autoregulatory mechanisms of the kidney attempt to maintain blood flow and GFR (think myogenic and tubuloglomerular feedback),
[edit] Intrinsic AKI
- There are many causes of intrinsic AKI, so we classify them by their histological location: tubules, interstitium, vasculature, or glomerulus.
[edit] Tubular intrinsic AKI
- Tubular injury often occurs by way of inschemia but may also occur because of specific renal toxins that target tubular cells.
- Tubular injury from ischemia is an extension of prerenal injury and occurs in four distinct stages: initiation, extension, maintenance, and recovery.
- Initiation: tubular cells demonstrate a severe depletion of ATP.
- Extension: microvascular congestion and inflammation occur.
- Maintenance: cells continue to repair, migrate, and proliferate to maintain tubular integrity.
- Recovery: GFR improves as cellular differentiation continues and normal cellular and organ function continues
- Tubular injury causes bleb formation on the apical membrane, loss of brush border, loss of surface membrane proteins, reduced polarity, loss of tight junctions, cell detachment, cast formation in the distal tubule (causing obstruction), and leakage of filtrate back into the interstitium.
- Renal toxins can act directly or indireclty.
- Direct damage to tubular epithelial cells: aminoglycocides, radiocontrast, and cisplatin (a cancer drug)
- Indirect damage via decreased blood flow: NSAIDs, cyclosporine, radiocontrast.
- Cocaine and HGM-CoA reductase inhibitors can cause skeletal muscle damage, releasing heme which acts as a sort of endogenous toxin to tubular cells.
- Precipitatory injury can occur when solutes or metabolites precipitate becasue of pharma: acyclovir, sulfonamides, ethylene glycol (calcium oxalate metabolite), methotrexate, and multiple myeloma light chains.
- Patients with comorbidities like diabetes mellitus are far more likely to come down with pharam-induced precipitatory toxin damage.
[edit] Interstitium intrinsic AKI
- Injury to the interstitium of the kidney is sometimes called acute interstitial nephritis (AIN).
- Acute interstitial nephritis is characterized by invasion of T cells, monocytes, and macrophages.
- These patches of inflammation can be diffuse or patchy.
- When chronic, AIN leads to interstitial scarring.
- AIN can be induced by drugs, infections, and auto-immune responses:
- Drugs: penecillins, cephalosporins, sulfonamides, and NSAIDs.
- Infections: bacterial and viral infections
- Autoimmune responses: systemic or localized to the kidneys
[edit] Vascular intrinsic AK
- Vascular injury to the renal tissue occurs at the micro or macro vascular level.
- Microvascular damage is usually due to thrombocytic purpura, sepsis, hemolytic uremia syndrome, and HELLP (hemolysis, elevated liver enzymes, and low platelets).
- Macrovascular damage can occur due to atherosclerosis, especially when plaque is dislodged during surgeries.
[edit] Postrenal AKI
- Postrenal AKI is generally caused by some sort of blockage, either within the tract, pressing on the tract, or a functional blockaged like denervation of the bladder.
- Intraluminal obstruction can be caused by "renal calculi", papillary necrosis, or blood clots in addition to drug precipitation.
- 'Calculus - a hard lump produced by the concretion of mineral salts; found in hollow organs or ducts of the body; "renal calculi can be very painful"'
- Drugs that precipitate or cause precipitation of solutes: uric acid, calcium oxalate, acyclovir, sulfonamide and methotrexate, as well as myeloma light chains.
- Postrenal AKIs initially are able to maintain the GFR (by way of afferent arteriole dilation ) in spite of the backpressure at the Bowman's space. However, at some point the back pressure overwhelms the ability of the afferent arterioles to compensate and the cortex begins to lose blood flow. Eventually, glomeruli are not well perfused and the tissue becomes injured by ischemia.
[edit] Clinical findings
- Clinical findings in AKI (acute kidney injury) are "protean" and are often not apparent until late in the course of kidney injury.
- Protean: "Exceedingly variable; readily assuming different shapes or forms; Of or pertaining to Proteus; characteristic of Proteus" per wiktionary
- Often, AKI is diagnosed by lab as the patient could be asymptomatic or have non-specific symptoms.
- Symptoms of AKI can include:
- anorexia
- fatigue
- nausea and vomiting
- pruritus: itching
- decline in urine output or dark-colored urine
- asterixis: "Asterixis (also called the flapping tremor, or liver flap) is a tremor of the wrist when the wrist is extended (dorsiflexion), sometimes said to resemble a bird flapping its wings." per wikipedia
- myoclonus
- pericardial rub
- volume overload leadning to:
- shortness of breath and dyspnea on exertion
- peripheral edema,
- pulmonary crackles and
- jugular venous distension
[edit] Diagnosis
- A thorough history and physical can be very useful in diagnosing and treating AKI.
- To achieve the optimal therapeutic plan, one should systematically evaluate all three categories of AKI: prerenal, intrinsic, and postrenal.
- Generally, one rules out pre and postrenal injury before evaluating intrinsic renal state.
- Laboratory values are especially important in diagnosis and treatment of AKI.
- Laboratory values can help determine how rapidly therapy should be commenced because kidney injury is usually clinically silent as it progresses.
- There are a series of tests that should be run for AKI assessment and diagnosis:
- a renal panel
- a CBC
- urine dipstick
- urine microscopy
- Urine microscopy should be performed on a fresh urine sample as etiology-indicating cellular elements can degrade quickly.
- a renal ultrasound
- To reveal blockages like stones.
[edit] CBC and FE (fractional excretion)
- One should pay particular attention to the Na, K, Cl, and creatinine values of the CBC and calculate the FENa (the fractional excretion of Na).
- FENa = (Urine Na * Urine creatinine / Plasma Na * Plasma creatinine) * 100
Why does it make sense that we multiply the concentrations? Won't this augment the change in FENa given a change in either Na or creatinine concentration?
- The value of FENa can guide the etiology of the AKI:
- Recall that in a healthy state, urine Na will be low and urine creatinine will be high. Also, plasma Na will be high and plasma creatinine will be high.
- Note that FECl is sometimes used when the patient is alkalotic because FENa will be elevated due to HCO3- secretion at the kidney coupled with Na secretion.
I expect that Na in the urine will increase when the kidney is injured in a way that damages the tubule and anything having to do with reabsorption. I expect that Na in the urine will decrease when the kidney is injured in a way that damages the vasculature or glomerulus and thus decreases the GFR.
- Let's examine the change in FENa for each type of AKI:
- Prerenal: FENa is decreased because the GFR will decrease and thus Na in the urine will decrease as there is less flow and more time to reabsorb the Na.
- Intrinsic:
- Vasculogenic intrinsic AKI: FENa will be decreased because the GFR will be depressed and thus Na in the urine will decrease as there is less flow and more time to reabsorb the Na.
- Glomerulogenic intrinsic AKI: FENa will be decreased because the GFR will be depressed and thus Na in the urine will decrease as there is less flow and more time to reabsorb the Na.
- Tubulogenic intrinsic AKI: FENa will be elevated because the tubule cells are less able to reabsorb Na from the filtrate.
- Interstitial intrinsic AKI: FENa will be elevated because the interstitial fluid is infiltrated with lymphocytes and all sorts of signals such that the expected gradient that helps tubule cells reabsorb Na is disrupted and urine Na is increased.
**Postrenal AKI: FENa is elevated because ....
- Decreased FENa occurs when AKI is due to prerenal issues or issues with vasculature or the glomerulus.
- Increased FENa occurs when AKI is due to postrenal issues or issues with the tubule or interstitium.
- So, the dividing point as to when AKI will result in increased or decreased AKI is at the glomerulus / vasculature.
[edit] Prerenal azotemia
- Prerenal azotemia (high levels of nitrogen containing compounds) is one of the most common etiologies of renal dysfunction.
- Common patient histories for prerenal azotemia include:
- vomiting, diarrhea, poor oral intake, and congestive heart failure
- CHF and other drugs can inhibit renal blood flow and thus cause azotemia.
- Common patient signs for prerenal azotemia include:
- tachycardia, systemic and / or orthostatic hypotension, and dry membranes.
- The FEurea is the measure of interest in assessing prerenal azotemia:
- Like FENa, FEurea = (Urine urea * urine creatinine / plasma urea * plasma creatinine) * 100
Do we use the creatinine terms as some sort of standard? Does creatinine excretion not change much during AKI? Why not consider only Na or only urea?
- FEurea less than 35 is inicative of AKI.
- Recall that FEurea will increase when there is excess BUN in the urine.
- We can also use plasma BUN to plasma creatinine ratio as an indicator of prerenal azotemia.
- A plasma BUN : plasma creatinine > 20 : 1 indicates prerenal azotemia.
[edit] Intrinsic AKI
[edit] Postrenal AKI
[edit] Treatment
- Stabilize extracellular comparment.
- Increase cardiac function to optimum.
- Give Na-Bicarb
- Identify and arrest drugs causing nephrotoxicity.
- Use preventative measures to reduce AKI:
- Educate patient about NSAIDs and other easy to get nephrotoxins.
- Monitor serum and urine Na, BUN, and creatinine.
[edit] Prognosis
- Prognosis is pretty good for prerenal AKI patients.
- Prognosis is worse for intrinsic AKI patients.
- started here on 03/30/11.
[edit] Acid-base balance
[edit] Objectives
- Define the following: acid, base, buffer, pH. Give the normal range of arterial blood pH and the limits compatible with life. Explain why constancy of pH is important.
- State the isohydric principle. List the important chemical buffers present in extracellular fluid, intracellular fluid, and bone.
- Write the Henderson-Hasselbalch equation for the bicarbonate/CO2 system. Write Henderson's equation for calculating [HCO3-] from [H+] and PCO2 measurements.
- Explain why the bicarbonate/CO2 system is so important.
- List the four simple acid-base disturbances. Describe for each: 1) the primary defect, 2) changes in arterial blood chemistry (pH, PCO2, and plasma [HCO3-]), 3) some common causes, 4) chemical buffering processes, and 5) respiratory and renal compensations.
- Given plasma electrolyte concentrations, calculate and interpret the anion gap.
- Given values for arterial blood pH, plasma [HCO3-], and PCO2 (or any two of the three), be able to identify the type of acid-base disturbance present.
[edit] Normal pH in arterial blood
- A normal pH is 7.4 (7.38-7.42), at which point the concentration of H+ is 40 (38 - 42) nmol / liter.
- Survivable pH is 7.0-7.6 which is considered acidosis and alkalosis, respectively.
- Note that this is a four fold change in H+ concentration.
- Death by change in pH occurs as a result of the changes in intracellular proteins upon change in pH.
[edit] Threats to pH of extracellular fluid
- There are two major forces that affect extracellular pH: oxidative phosphorylation and protein metabolism.
- Both of these processes produce sources of acid: oxidative phosphorylation produces CO2 and protein metabolism produces H2SO4 and HCl.
- Oxidative phosphorylation as a source of extracellular acid:
- Recall that oxphos dumps electrons onto oxygen and secretes this waste as CO2.
- Recall that CO2--via carbonic anhydrase--affects the extracellular levels of H+ and HCO3-.
- That is, as CO2 rises, more H2CO3 and H+ are found in the blood; the pH decrease.
- We say CO2 is a volitile acid because it indirectly affects H+ levels.
- 13-20 moles of CO2 are produced each day by oxphos.
- Protein metabolism as a source of extracellular acid:
- Recall that proteins are digested for energy and as a source of amino acids.
- Metabolism requires the removal and storage of the many hydrogens on the proteins and thus can generate H+.
- Met or Cys metabolism generates H2SO4.
- Lys or Arg metabolism generates HCl.
- We say protein metabolism generates fixed acids because it directly affects H+ levels.
- 40-60 moles of fixed acid are produced each day by protein metabolism.
[edit] Chemical buffers
- Recall that a chemical buffer is an acid (HA) and it's conjugate base (A-).
- There is an equilibrium among these two components as the hydrogen dissociates and reassociates: HA <-> H+ + A-
- The Ka describes the balance between the two entities (A- and HA): Ka = [H+][A-] / [HA]
- Recall the Henderson-Hassalbach equation which describes the pH in terms of the Ka and the amount of conjugate base (A-) present in a sample: pH = pKa + log([A-] / [HA])
- Recall that pKa = log(Ka) = log([H+][A-] / [HA])
- So the Henderson-Hasselbach equation takes the (log of the) expected balance ([H+][A-] / [HA]) and modifies it by adding (the log of) the ratio of conjugate base to conjugate acid.
- Chemical buffers of the body come exist in three compartments: ECF, ICF, and bone.
- Each of the buffers in these three compartments can add or remove H+ from the system in order to buffer the pH.
- The ECF contains HCO3- / CO2, plasma proteins, and inorganic phosphates.
- The ICF contains proteins, organic phosphates, some HCO3- / CO2.
- The bone contains pohosphate and carbonate salts.
[edit] Isohydric principle
- The isohydric principle says that at any given concentration of H+, all conjugate acid / base pairs are in equilibrium.
- This means that the pH can be determined from any pair of conjugate acid / base pairs using their pKa.
- pH = pKa + log([A-] / [HA])
- pH = 6.1 + log([HCO3-] / 0.03*PCO2)
- pH = 6.8 + log([HPO4] / [H2PO4])
- pH = pKHPr + log([Pr-] / [HPr])
- pH = pKa + log([A-] / [HA])
[edit] Negative feedback controls on endogenous acid production
- There is a negative feedback system that helps resist changes in systemic pH when endogenous acid production is high.
- This system may have an effect during vigorous activity or diabetic ketoacidosis.
- This negative feedback system is based on cellular enzymes that produce endogenous acid (think back to oxphos and protein metabolism as endogenous sources of acid).
- Named examples of acid-producing processes include ketoacidosis and lactic acidosis.
- Negative feedback of pH change is probably mediated by the inherent effect that a change in pH has on these enzymes that make endogenous acid: as as pH goes up, endogenous acid decreases (because the enzymes don't function as well at high pH) and as pH drops endogenous acid increases (because the enzymes work better at higher pH).
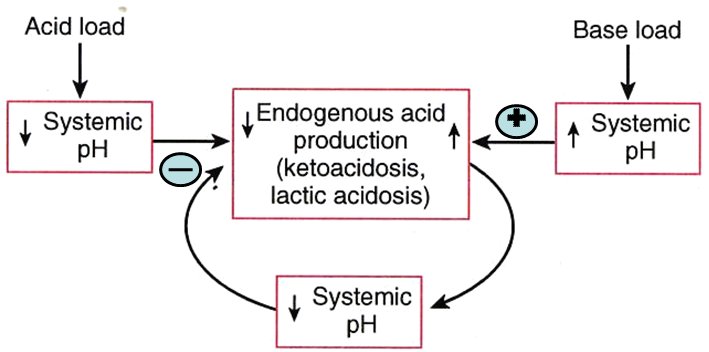
[edit] The importance of the HCO3 / CO2 buffer system
- The HCO3 / CO2 buffer has two characteristics that make it very effective: abundance of buffer pair molecules, an "open" system.
- Bicarb / CO2 is a good buffer because of it's abundance:
- There is 24 mM of HCO3 in the blood.
- There is 1.2 mM of CO2 in the blood and 13-20 moles produced each day.
- Having lots of molecules means that many molecules of H+ can be bound or unbound as needed.
- Bicarb / CO2 is a good buffer because it is an "open" system:
- Recall that CO2 can be breathed off at the lungs and HCO3 can be secreted by the kidneys.
- Because of these physiological connections to organs that "open" up to the external environment, the HCO3 / CO2 system is considered "open".
- Note that the lungs can respond quickly (within minutes) and the kidneys respond slowly (within days) to balance HCO3 / CO2.
[edit] HCO3 / CO2 equilibrium
- Recall the carbonic anhydrase driving equation: CO2 (aq) + H20 <-> H2CO3 <-> H+ + HCO3
- Recall that the amount of aqueous CO2 is always 0.03 * PCO2.
- This is useful because PCO2 is measurable in the clinical setting.
- When PCO2 is 40 mmHg (pretty normal), CO2 (aq) is 1.2 mM.
- So when we calculate pH based on this:
- pH = pKa + log([A-] / [HA])
- pH = 6.1 + log([HCO3 / [CO2])
- pH = 6.1 + log([HCO3 / 0.03 * PCO2)
- pH = 6.1 + log(24 / 1.2)
- The 20:1 ratio of HCO3 : aqueous CO2 is key to having a normal pH.
- pH = 6.1 + log(20)
- pH = 6.1 + 1.3
- pH = 7.4
- But there is a simpler way: the clinical equation.
- In clinical settings, logs are not really practical so we use an alternative equation.
- The clinical equation uses two values easily procured by labs (PCO2 and [HCO3]) to calculate the concentration of H+ ([H+]) which is compared to an expected value (40 nM) to determine if the pt is acidotic ([H+] higher than 40 nM) or alkalotic ([H+] lower than 40 nM).
- [H+] = 24 * PCO2 / [HCO3]
- Recall that PCO2 and [HCO3] are usually known so we are calculating to find [H+].
- We have an expectation as to what a normal [H+] should be (40 nM) and this equation tells us if [H+] is high (low pH, acidosis) or low (high pH, alkalosis).
[edit] The lungs as an opening to the environment
- Recall that the lungs can breath off CO2 and thus decrease the pH of the blood (shifts equation away from H+ + HCO3-).
- As ventilation increases, more CO2 is breathed off.
- Ventilation is stimulated by increased arterial PCO2 or decreased pH.
- Note that neither of these forces can cause at pt to ventilate to their full voluntary ventilation capacity; thus, one can hyperventilate themselves into passing out--because one can throw off acid-base balance by ventilating too rapidly so the body arrests auto-hyperventilation by blacking out.
[edit] 4 acid-base distrubances
- There are four acid-base distrubances: acidosis and alkalosis arising from respiratory or metabolic origins.
- Recall that there are three compensation mechanisms that are very fast, fast, and slow: chemical buffering of the blood by HCO3 / CO2 (very fast, seconds), respiratory compensation by breathing off CO2 (fast, minutes), and renal compensation by excreting H+ / HCO3 (slow, days).
- Note that in respiratory acid-base imbalances, the primary variable that has changed is CO2 (because the lungs can only increase or decrease this one variable).
- Therefore, compensation mechanisms in pulmonary malfunction will always attempt to change HCO3 in the same direction as CO2.
- Note that in metabolic acid-base imbalances, the primary variable that has changed can be H+ or HCO3- (because metabolic processes can cause a change in either acid or base production).
- Therefore, compensation mechanisms in metabolic malfunction can attempt to change the opposite factor (base in an acid-malfunction or acid in a base-malfunction) in the same direction or can attempt to counteract the changes to CO2 levels.
[edit] Respiratory acidosis
- Respiratory acidosis is defined as "any abnormal pulmonary function that results in CO2 accumulation".
- As CO2 accumulates the equation shifts to the right, toward H+ and HCO3.
- However, HCO3 production does not keep up with the shift so H+ > HCO3-.
- Common cause: hypoventilation
- Compensation:
- Recall that compensation mechanisms attempt to normalize the HCO3 / CO2 ratio by moving the opposite factor in the same direction; therefore as CO2 increases, the compensation mechanisms will attempt to increase HCO3, also.
- Chemical buffering: mostly achieved by proteins binding H+ in cells (think Hb).
- Pulmonary compensation: none, lungs are the problem
- Renal compensation: raise the HCO3 content of the blood; attempt to restore a HCO3 / CO2 ratio of 20:1.
- This is an attempt to modify the HCO3 / CO2 ratio by increasing HCO3.
- Acute respiratory acidosis results in a renal compensation of about 1 mEq / L of HCO3 production for every 10 mmHg increase in PCO2.
- Chronic respirartory acidosis results in a renal compensation of about 4 mEq / L of HCO3 production for every 10 mmHg increase in PCO2.
[edit] Respiratory alkalosis
- Definition: "any abnormal pulmonary function that results in CO2 deficiency".
- As CO2 is released, the equation shifts to the left, decreasing H+ and leaving HCO3 to cause alkalosis.
- Common causes: hyperventilation
- Compensation:
- Recall that compensation mechanisms attempt to normalize the HCO3 / CO2 ratio (to 20:1) by changing the non-effected value in the same direction.
- In respiratory alkalosis, the CO2 level is too low so the body attempts to compensate by decreasing the amount of HCO3-.
- Chemical buffering: mostly achieved by release of H+ by intracellular proteins.
- Pulmonary compensation: none, lungs are the problem.
- Renal compensation: decrease the plasma HCO3- levels
- decrease HCO3- production, increase HCO3- secretion
[edit] Metabolic acidosis
- Definition: "any abnormal function that results in a gain of acid or loss of base (excepting the gain of H2CO3)".
- Recall that while pulmonary acid-base imbalances result from changes in CO2,] metabolic acid-base imbalances can result from acid or base changes.
- Adding an acid makes the pt acidotic and pushes the HCO3 / CO2 reaction toward CO2.
- Removing a base makes the pt acidotic and pulls the HCO3 / CO2 reaction toward H+ / HCO3-.
- Common causes:
- renal failure,
- excessive intake of nonvolatile acids,
- excessive production of nonvolatile acids (ketoacidosis, lactic acidosis, ingestion of acidosis, ingestion of acidifying agents),
- poisons (salicylate, methanol, ethylene glycol),
- loss of bicarbonate (excessive urinary excretion of bicarbonate [renal tubular acidosis], diarrhea)
- Compensation:
- Chemical buffering: half of the buffering occurs in the cells and bone, HCO3- is the main ECF buffering base.
- Pulmonary compensation: prompt hyperventilation to lower the PCO2; cannot completely compensate, however.
- Note that in the case of added acid, this would breathe off the elevated CO2, decreasing acid.
- Note that in the case of removed base, this would provide counter force along the equation axis to keep some of the acid in CO2 form.
- Renal compensation: increased H+ secretion, increased ammonia synthesis, increased bicarb reabsorption / production.
[edit] Anion gap
- Recall that metabolic acidosis can result from a depletion of HCO3.
- The chief purpose of calculating the anion gap is to determine the source of HCO3 depletion; that is, to determine the etiology of metabolic acidosis.
- Recall from general chemistry that charges in a solution like to be in equilibrium; the body maintains a certain equilibrium of positively charged ions (cations) to negatively charged ions (anions).
- In general, the anions and the cations are in equilibrium (as with all solutions).
- We can simplify this to a short equation if we only take into account the major ions:
- [Na+] = [Cl-] + [HCO3-] + [unmeasured anions]
- The anion gap is the difference in the cations and the anions.
- Note that we drop the unmeasured anions term because...well...they are unmeasured so we don't know the value.
- Unmeasured anions include lactate, ketones, proteins, phosphate, citrate, and sulfate to name a few.
- These will become causes of acidosis when they are aberrently elevated.
- Anion gap = [Na+] - [Cl-] - [HCO3-]
- Normal anion gap = 140 - 105 - 24 = 11 mEq / L
- This makes sense because Na is the highest concentration of cations, Cl is the major important anion, and HCO3- is the variable.
- Note that we drop the unmeasured anions term because...well...they are unmeasured so we don't know the value.
- So what does an increased anion gap mean?
- Recall that a normal anion gap = 140(Na+) - 105(Cl-) - 24(HCO3-) = 11 mEq / L.
- An increase in the anion gap means that there is less HCO3 to subtract from the Na or there is less Na from which to subtract the HCO3.
- Metabolic acidosis generates an increased anion gap because of a deficiency of HCO3 secondary to over production of metabolic acids like lactic acid, ketone body acids, or toxins.
- So what does a normal anion gap mean?
- It could mean that there is no acid-base problem. :)
- It could mean that the metabolic acidosis is caused by renal tubular acidosis, here's why:
- When HCO3- is lost (loss of a base leads to acidosis) at the renal tubule (perhaps because of diarrhea in which high volume requires lots of HCO3- secretion to maintain filtrate pH; perhaps because of poor HCO3 reabsorption at the PCT) it can be exchanged for Cl-.
Is this exchange an active exchange like Na for K in water balance or is it indirect like "well because HCO3 wasn't reabsorbed and yet the filtrate must be ionically balanced, Cl gets reabsorbed"?
- When HCO3- is exchanged for CL- at the renal tubule, the anion gap doesn't change, yet HCO3- is being depleted. That is, when metabolic acidosis occurs without an anion gap, one knows that the renal tubule is the source of HCO3 depletion.
- Metabolic acidosis generates a normal anion gap because of a deficiency of HCO3 secondary to over excretion of HCO3 at the renal tubule.
- Common causes of anion-gap acidosis:
- MULEPaKS
- Methanol
- Uremia (renal failure, urine in the blood)
- Lactic acid
- Ethylene glycol
- pAldehyde
- Ketone body acids
- Salicylates
[edit] Metabolic alkalosis
- Definition: "any abnormal function that results in a gain of base or loss of acid (especially the gain of bicarbonate but not the loss of H2CO2)".
- Recall that gain of bicarb or another base will push the HCO3 / CO2 reaction toward CO2.
- Recall that loss of acid will drag the HCO3 / CO2 reaction toward H+ / HCO3.
- Common causes:
- Acid loss: vomiting (stomach juices have lots of H+), hyperaldosteronism (excessive H+ loss at the kidney), hypokalemia (excessive H+ loss at the kidney via the H-K exchanger that will reabsorb K by sacrificing H+ to the filtrate)
- Base gain: excessive alkali intake
- Compensation:
- Chemical buffering: 1/3 in the cell compartment
- Pulmonary compensation: hypoventilation to increase PCO2 (but cannot fully compensate)
- Recall that HCO3- cannot be generated as quickly as acid when the HCO3 / CO2 equation is shifted toward H+ / HCO3.
- So increasing PCO2 will compensate for excessive acid loss by pushing the equation toward H+ / HCO3 and thus regenerating some of the acid.
- So increasing PCO2 will compensate for base gain by pushing the equation toward H+ / HCO3 and regenerating acid (faster than it regnerates HCO3) to counter the excess in base.
- Renal compensation: reabsorb less PCO3 and therefore lower the plasma HCO3.
- Note that this will compensate for increased base by decreasing another base: HCO3.
- Note that this will compensate for decreased acid by equalizing the ratio of acid to base.
[edit] Davenport diagram: A magic, visual explanation

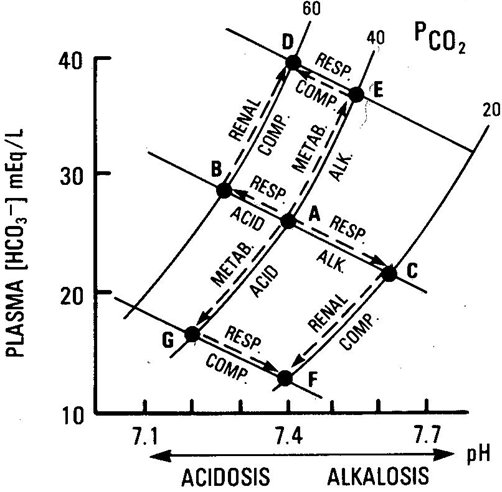

[edit] Examples of acid-base values
| pH | PCO2 (mmHg) | [HCO3-] (mEq / L) | State | |
|---|---|---|---|---|
| 7.08 | 49 | 14 | Respiratory acidosis + Metabolic acidosis (pulmonary disease -> low O -> high lactic acid) | |
| 7.32 | 28 | 14 | Metabolic acidosis | Respiratory compensation |
| 7.40 | 40 | 24 | Normal | NA |
| 7.51 | 49 | 38 | Metabolic alkalosis | Respiratory compensation |
| 7.53 | 20 | 16 | Respiratory alkalosis | Renal compensation |
| 7.62 | 20 | 20 | Respiratory alkalosis | Respiratory compensation |
- stopped here on 03/30/11.
- started here on 03/30/11.
[edit] Renal regulation of acid-base balance
[edit] Objectives
- Describe the three processes involved in urinary acidification: reabsorption of filtered bicarbonate, formation of titratable acid, and excretion of ammonia.
- Explain why most of the hydrogen ions secreted by the renal tubules are not excreted. Explain why excretion of titratable acid and ammonia (as NH4+) adds new bicarbonate to the blood. Be able to calculate net acid excretion from measurements of urinary ammonia, titratable acid, and bicarbonate excretion.
- Discuss the factors that influence renal secretion and excretion of hydrogen ions.
- Describe the renal compensation for each kind of acid-base disturbance.
[edit] Renal acid excretion
- There are two main acids excreted by the kidney: ammonia (NH4+) and titratable acids (TA)
- In this context, titratable can be defined as "able to accept a proton"
- There is one main base excreted by the kidney: bicarbonate (HCO3-)
- Therefore, the net acid secretion by the kidney is the acids - the base:
- Renal acid secretion = TAs + NH4 - HCO3
- Normal acid secretion = 70 = 24 + 48 - 2 (mEq / day)
- The kidney can adjust acid secretion over a wide range
- Note that excreting acid is equivalent to adding new bicarbonate to the blood.
- Note that the amount of free H+ in the urine is very small.
- Acid excretion pathology:
- Acid secretion is elevated in diabetes mellitus:
- Diabetes mellitus renal acid secretion = 700 mEq / day = 200 (mEa TAs) + 500 (mEq NH4+) - 0 (mEq HCO3-)
- Acid secretion is most often elevated when consuming mixed meat / vegetable diets.
- Vegetarians excrete less acid.
- Acid secretion is elevated in diabetes mellitus:
[edit] Acid-base regulation overview
- Recall from the Acid-base balance lecture that there are three regulation mechanisms: chemical buffering, pulmonary compensation, and renal compensation.
- We will no discuss the kidney's function in acid-base balance more fully.
- Recall that we previously discussed the kidney's ability to secrete H+ and / or HCO3- to rebalance the pH.
- The kidney is also capable of generating novel HCO3 and secreting titratable acids made of sulfates, chlorides, and phosphates.
- There are three processes involved in acidifying the urine:
- reabsorption of filtered bicarbonate (the more that is reabsorbed, the more acidified the urine)
- formation of titratable acid (to bind H+ cations)
- excretion of ammonia
- The production of TAs and the secretion of NH4 / NH3 results in novel bicarbonate added to the blood to replace bicarb consumed in buffering against increased acids.
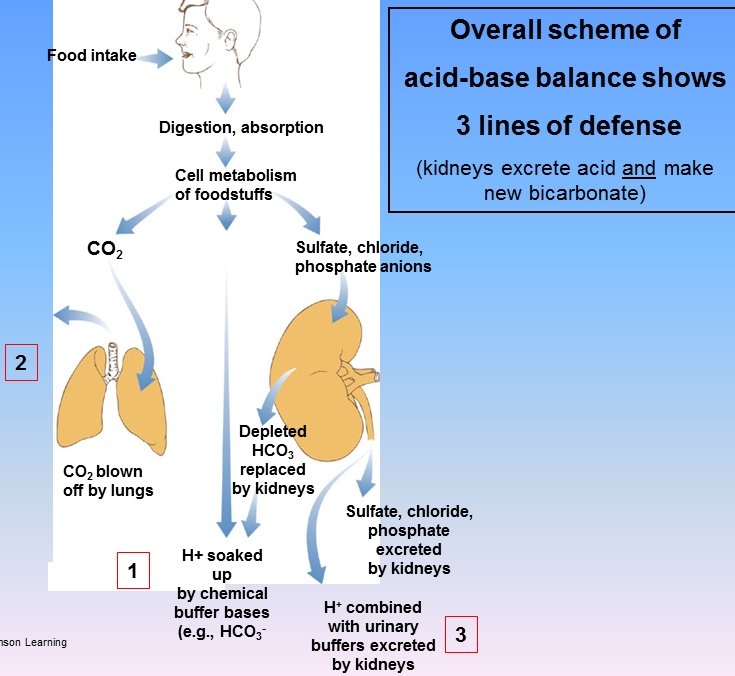
[edit] Reabsorption of filtered bicarbonate
- Normally, 99.9% of filtered bicarbonate is reabsorbed by the nephron.
- When plasma HCO3 is low, there is 0 excretion.
- Note, however, that there is a threshold at which the flow rate (and therefore the amount of filtered HCO3) is so high that it cannot all be reabsorbed.
- So, this first step in urine acidification is pretty constant: the HCO3 population of base in the urine is almost always, almost completely removed and does not increase the plasma HCO3- level.

[edit] Mechanism for reabsorption of filtered bicarbonate
- As with so many things, bicarbonate is reabsorbed using the Na gradient.
- Bicarbonate from the filtrate is reabsorbed using two Na exchangers, one on the apical membrane and another on the basolateral membrane.
- Recall that HCO3- requires a transporter to cross the membrane but CO2 can diffuse across.
- Recall that in RBCs we use CA (carbonic anyhdrase) to convert HCO3- to CO2 so it can diffuse over the membrane.
- Recall that in RBCs we use a HCO3-Cl exchanger to move HCO3 in and out of the cell.
- So, in order to facilitate HCO3- reabsorption we convert it to CO2:
- Recall: H+ + HCO3- <-> H2CO3 <-(CA)-> H20 + CO2
- Filtered HCO3- exists as HCO3- in the filtrate; so we need to provide H+ to get the reaction to head toward CO2.
- A Na-H exchanger on the apical membrane reabsorbs Na and moves H+ into the filtrate.
- HCO3- + H+ -> H2CO3 -(CA)-> CO2 + H20
- CO2 enters the tubule cell.
- CO2 + H20 (both in the cell) -(CA)-> H2CO3 -> H+ + HCO3
- A Na-HCO3 cotransporter on the basolateral surface moves Na and HCO3 into the plasma.
- The aforementioned, apical Na-H exchanger moves H+ into the filtrate (to facilitate another conversion of HCO3 into CO2).

[edit] Formation of titratable acid
- Recall that the type A intercalated cells of the collecting duct secrete H+.
- Recall that H+ can readily cross back into the lining cells / interstitial fluid.
- In order to trap H+ in the filtrate, tubular cells of the nephron secrete titratable acids (that is, acids that can accept another H+).
Where in the nephron does TA secretion occur?
- So, as TA secretion increases, the pH of the filtrate (urine) decreases.
- The pH of filtrate decreases as it passes along the nephron.

[edit] Mechanism for formation of titratable acid
- Note that formation of titratable acid generates NEW bicarbonate for the blood.
- Recall the Na-H exchanger on the apical surface of proximal tubule cells that was used to reabsorb HCO3-.
- Recall the Na-HCO3- cotransporter on the basolateral surface of proximal tubule cells that was used to reabsorb HCO3-.
- The same source of H (the apical Na-H) provides H+ to protonate filtered TA-salts (like HPO4-2Na) to titratable acids (like H2PO4-1Na).
- The exchange of Na for H (Na moves into the cell, H+ moves into the filtrate) requires an intracellular supply of H+.
- CA provides the H+ by combining CO2 and H20 to generate H2CO3 and then H+ and HCO3-.
- As the CA-produced H+ is moved into the filtrate in exchange for Na, the CA-produced HCO3- is moved into the blood along with Na (via the aforementioned Na-HCO3 cotransporter).
- Note that production of titratable acids uses CA and thus generates NEW bicarbonate for the plasma.

[edit] Excretion of ammonia
- First, note that when we say "ammonia" we mean both ammonium ion (NH4+) and the free base NH3.
- Recall that ammonium ion and ammonia free base live in equilibrium: NH4+ <-> NH3+ + H+
- Recall that pH can be calculated by the Henderson-Hasselbach equation if the pKa is known for an conjugate acid / base pair.
- In this case, the pKa of NH4+ / NH3+ is 9.0.
- pH = pKa + log([A-]/[HA])
- pH = pka + log([NH3]/[NH4])
- Normally, urine has a pH around 7 (though it can vary from 4.4 to 8).
- 7.0 = 9.0 + log([NH3]/[NH4])
- -2 = log([NH3]/[NH4])
- antiLog(-2) = [NH3] / [NH4]
- 10-2 = 1/100 so the ratio of NH3 to NH4 is 1:100.
- That is NH4 >>> NH3.
- So there is very little free H+ in the urine!
- Ammonia secretion by the nephron accounts for 2/3 of the H+ secreted by the kidney.
- So it is an important part of the kidney's acid-base regulation response.
- Ammonia is produced by proximal tubule cells from amino acid metabolism, especially glutamine.
- Recall that the goal is to reduce acid and increase bicarbonate.
- Note that while titratable acid production can generate new bicarbonate, it requires titratable salts (like HPO4-2Na, HSO4-Na) which are of limited supply in the filtrate.
- Therefore acid secretion by NH4/NH3 secretion is the primary method secreting lots of acid and producing lots of HCO3.
- Ammonia synthesis can be increased over a series of days and is a life saving adaptation.
[edit] Mechanism for excretion of ammonia
- Recall that we can help balance pH by secreting acid and that NH4/NH3 (ammonia) molecules are the primary acid secreted in the nephron proximal tubule.
- We will look at this process as two steps: secreting ammonia acid and generating new HCO3.
- Secreting ammonia acid:
- Recall that glutamine is the primary source of nitrogen for generating ammonia for secretion.
- Glutamine can be converted to 2 NH4 molecules by intracellular enzymes of the tubule cells.
- Here we introduce a third surface membrane: the Na-NH4 exchanger: the apical Na-NH4 exchanger moves Na from the filtrate into the cell and NH4 from the cell into the filtrate.
- Simple enough: convert glutamine into two NH4 molecules and use the Na-NH4 exchanger to secrete NH4 into the filtrate.
- Generating new HCO3 for the plasma:
- Recall that we can generate HCO3 if we can find some place to dump the H+ generated by the CA reaction.
- Recall that glutamine can be converted to alpha-ketoglutarate which--with the addition of hydrogens--can be converted to glucose.
- So with alpha-ketoglutarate (from glutamate) as an H+ acceptor (and because glucose will be happy to move out of the cell into the plasma) we can drive the CA reaction and generate HCO3.
- Once the CA reaction has generated HCO3, it can follow Na into the plasma via the same, basolateral Na-HCO3 cotransporter as was used in HCO3 reabsorption and titratable acid production.

[edit] Most H+ secretion occurs in the proximal tubule
- Recall that most of the NH4/NH3 secreted by the nephron occurs in the proximal tubule.
- Recall that most HCO3 is reabsorbed in the proximal tubule.
- There is little change in the filtrate pH in the proximal tubule because:
- most of the secreted acid reacts with HCO3 to form H2CO3 and
- the proximal tubule has a leaky epithelium through which hydrogen ions and HCO3- can pass
- The collecting duct is the site of the largest blood-urine pH gradients.
- This makes sense because it has a tight epithelium that does not allow the passage of water, H+, or HCO3-.
- Recall that type A intercalated cells actively secrete H+ into the filtrate to combat acidosis.
- Recall that type B intercalated cells actively secrete HCO3- into the filtrate to combat alkalosis.
- Highest pH blood-urine gradient is 7.4 to 4.5.
- What is the increase in [H+] over this gradient?
- 7.4 - 4.5 = 2.9
- So 102.9</sub> ~= 1000; so the urine has 1000-fold higher concentration of H+.
[edit] Factors that affect H+ secretion and excretion at the kidney
- Intracellular pH
**....
- Arterial PCO2
**Arterial PCO2 must be maintained such that CA can't function too quickly or PCO2 levels would rise too high.
- Carbonic anhydrase activity
**CA has an inherent maximum functionality; this limits the amount of HCO3 that can be produced for release into the plasma.
- Note that acetazolamide inhibits carbonic anhydrase and can thus be used as a diuretic as decreased production of intracellular H+ (from the CA reaction) means less H+ for the apical Na-H+ exchanger which means less Na reabsorption which means less water reabsorption.
- Sodium reabsorption
- Increased Na reabsorption occurs by exchange with H+ ions (into the filtrate) which means that increased Na reabsorption leads to increased acid secretion.
- Plasma potassium concentration
- If K is low, a K+-H+ exchanger moves H+ from plasma into the tubular cells and K+ from the tubular cells into the plasma.
**Rise of H+ in the tubular cell means that more H+ will be secreted.
- Aldosterone
- Elevated plasma aldosterone leads to increased Na+ reabsorption and increased apical H+ ATPase activity (which secretes H+).
**This causes increased H+ secretion....
- Availability of buffers
- As buffer availability increases, less acid needs to be secreted and less HCO3 needs to be produced.

[edit] Timeline of acid-base compensation mechanisms
- Recall that the three acid-base compensation mechanisms have differing time frames:
- chemical buffering is very fast (seconds)
- pulmonary compensation is fast (minutes to hours)
- renal compensation is slow (hours to days)

- stopped here on 03/30/11.
- started here on 04/04/11.
[edit] Sexual differentiation and the HPG Axis
[edit] Learning objectives
- Prof will take exam questions from objectives.
[edit] Sexual differentiation
- Genetics is determined at fertilization.
- XY = male
- XX = female
- The sperm has either an X or a Y and donates it to the X-containing ovum.
- There are many levels of sexual differentiation:
- establishing the genetic sex
- differentiation of the gonads
- differentiation of the internal reproductive organs
- differentiation of the external genitalia
- gender role
- gender identity
[edit] Differentiation of the gonads
- As an embryo develops, the gonads become the source of gender hormones:
- In males, the gonads become the testes and provide testosterone and dihydrotestosterone.
- In females, the gonads become the ovaries and provide estrogen.
- The gonads take their developmental cues from their genotype as to how it should develop and what hormones it should produce.
- An XY gonad has a Y chromosome with the Sex-determining region Y (SRY).
- SRY is also called testis determining factor (TDF).
- SRY is the master switch that causes differentiation to head toward male.
- SRY encodes a transcription factor that is part of the high mobility group (HMG) family.
[edit] SRY and PAR on the Y chromosome
- The PAR (psudoautosomal region) of the Y chromosome is a well conserved area that allows the Y chromosome to pair with the X chromosome for cell division.
- PAR is at the very distal area of the short arm of the Y chromosome.
- SRY is located just proximal to the PAR and is considered part of the sex determining region.
- Two diseases are associated with SRY:
- SRY defects lead to XY females; Swyer syndrome.
- Translocation of the SRY region from the Y chromosome to the X chromosome yields XX males; XX male syndrome.
[edit] Differentiation of the internal genital ducts
- Initially, embryos initially have a set of undifferentiated gonads and both Wolffian ducts and Mullerian ducts.
- The ducts become the transporters of sperm or egg.
- Wolffian ducts mature into the epididymis and vas deferens.
- Mullerian ducts mature into the oviduct, uterus, and upper part of the vagina.
- Based on the genotype of the gonads (that is, the presence or absence of SRY), the gonads will begin to express hormones.
- Testes produce AMH (anti-Mullerian hormone), testosterone, and dht (dihydrotestosterone).
- AMH causes involution of the Mullerian ducts and testosterone causes proliferation of the Wolffian ducts.
- Ovaries produce no hormones embryonically.
- A lack of hormones allows Wolffian ducts to involute and causes Mullerian ducts to proliferate.
- Testes produce AMH (anti-Mullerian hormone), testosterone, and dht (dihydrotestosterone).
- The presence of hormones from the gonads determines the differentiation of the internal genitalia.
- If SRY is present:
- AMH, test, and testosterone are produced by the developing gonads
- Anti-Mullerian hormone (AMH) is responsible for degeneration of the female-associated Mullerian ducts in males
- We say that the Mullerian ducts involute; involute: "rolled inwards spirally" per [www.biology.lsu.edu/heydrjay/ThomasSay/terms.html LSU Biology]
- Gonads differentiate into testes.
- If SRY is not present:
- No hormones are produced by the developing gonads
- The Wolffian ducts atrophy.
- Gonads differentiate into ovaries.
- Note that female seems to be the default gender.
[edit] Swyer syndrome
- Recall that Swyer syndrome results from a SRY defect in an XY patient.
- Swyer syndrome is considered a type of hypogonadism because the expected male gonads did not develop.
- Swyer syndrome is considered a "pure" gonadal dysgenesis because there is no chromosomal defect; that is, they have a normal karyotype.
- Gonads are underdeveloped and are often referred to as "streaks".
- Not that though the gonads do not develop correctly in Swyer syndrome, the internal and external genitalia do develop normally.
- Note, however, that puberty does not occur normally so external genitalia do not mature through puberty.
- Patients with Swyer syndrome are often treated with estrogen and progesterone replacement therapy.
[edit] Klinefelter's syndrome
- Klinefelter's syndrome results from a 47 XXY genotype.
- XXY genotype results in poorly developed testicles.
- Underdeveloped testicles can result in non-masculine features and pro-feminine features:
- Non-masculine: poor beard growth, poor chest hair growth, frontal hair growth (lack of frontal balding), small testicular size
- Pro-feminine features: narrow shoulders, wide hips, breast development, female-like pubic hair growth
- 1:1000 males has Klinefelter's syndrome
[edit] Differentiation of external genitalia
- Like gonads and ducts (internal genitalia), the external genitalia begin in a bipotent state from which they can develop into either male or female external genitalia.
- External genitalia are signaled to develop by the presence or absence of androgens--particularly DHT.
- Male external genitalia develop in the presence of DHT.
- Female external genitalia develop in the absence of DHT.
Listen for how much anatomy we need to know.
- Said he won't ask specific details; just wants us to know that the pre-anatomy has bipotential.
- One exam question from everything previous to this comment.
[edit] Gender role
- Gender role is the gender presented by an individual to society.
- Can be independent from anatomy and chromosomes.
- Gender role can be expressed through name, clothing, physical appearance, family role, occupation, and behavior.
No exam questions on this.
[edit] Gender identity
- Gender identity is the internal conviction of one's own gender.
- We do not currently understand all the factors and complexity of gender identity.
- There is an interesting, intimate relationship between nature and nurture as it relates to development of role identity.
- Think prenatal androgen exposure, family beliefs, appearance of the genitalia, and medical / surgical experiences.
No exam questions on this.
- One exam question from everything after this comment.
[edit] Key concepts of the HPG axis
- The HPG axis is the hypothalamus-(anterior)pituitary-gonad axis.
- Note that the HPG axis also includes some activity from the cortical regions of the brain (the higher-function centers of the brain).
- Some examples of higher brain centers that affect the hypothalamus are the visual, olfactory, pineal and stress centers.
- The hypothalamus contributes to the HPG axis by releasing GnRH.
- GnRH binds to receptors on the gonadotropes of the anterior pituitary.
- The gonadotropes of the anterior pituitary contribute to the HPG axis by releasing leutinizing hormone (LH) and follicle stimulating hormone (FSH).
- The gonads contribute to the HPG axis by secreting sex steroids and peptide hormones.
- The gonads also release inhibin which feeds back on the anterior pituitary to reduce LH and FSH release.
- The gonads are also the site of germ cell production and maturation.
- Testosterone and estrogen from the gonads feed back on the anterior pituitary and the hypothalamus to reduce LH / FSH and GnRH release, respectively.

[edit] HPG axis in males
- In males, the hypothalamus releases GnRH to affect gonadotropes of the anterior pituitary.
- Upon GnRH signaling, gonadotropes of the anterior pituitary release LH and FSH to affect the testicles.
- LH and FSH negatively feedback on the hypothalamus, too.
- Upon LH / FSH signaling, the leydig and sertoli cells of the testicles release testosterone and inhibin.
- Testosterone triggers spermatogenesis and negatively feeds back on the anterior pit and hypothalamus.
- Inhibin inhibits the anterior pituitary.
- Note that testosterone is bound by ABP (androgen binding protein) in the blood.
http://www.uptodate.com/contents/images/ENDO/5463/HPG_axis_PI.jpg?title=HPG+axis+PI
[edit] HPG axis in females
- In females, the hypothalamus releases GnRH to affect gonadotropes of the anterior pituitary.
- Upon GnRH signaling, gonadotropes of the anterior pituitary release LH and FSH to affect the ovaries.
- Note that LH / FSH don't negatively feed back on the hypothalamus like they do in the male.
- Upon LH / FSH signaling, granulosa cells of the ovaries release estradiol, progesterone, inhibin, and activin.
- Estradiol and progesterone go on to affect target cells.
- Estradiole and progesterone have opposite feedback effects on the anterior pit and hypothalamus depending on the phase: positive feedback in the follicular phase and negative feedback in the luteal phase.
- This makes sense because females need to make and mature oocytes on a cycle each month.
- Activin increases FSH production and release and systemically increases proliferation.
- Inhibin decreases FSH production and release and systemically decreases proliferation
- Estradiol and progesterone go on to affect target cells.

[edit] Higher centers
- The HPG axis is affected by stress, sight, smell, and emotion.
- These emotions can generate inhibitory or stimulatory signals.
No exam questions on this.
[edit] Neurotransmitters that affect the HPG axis
- There are LOTS of NTs that affect the HPG axis: norepinephrine, dopamine, epinephrine, acetylcholine, endorphins / opioids, neuropeptide Y, leptin, serotonin, cholecystokinin, GABA-major inhibitory NT.
[edit] Hypothalamus
- The hypothalamus releases GnRH at 70-90 minute intervals; we call this autorythmicity.
- GnRH is a chromosome 8, 10mer peptide with a very short half-life--around 3 minutes.
- The cells that secrete GnRH are neurons located in the arcuate nucleus of the medial basal hypothalamus (MBH).
[edit] Immortalized GnRH secreting neurons
What is the point of this slide?
[edit] Pituitary Gonadotropins
- FSH and LH are released by gonadotrophs of the anterior pituitary.
- FSH and LH are alpha-beta in structure; alpha is identical but beta is unique.
- This won't be tested.
- Gonadotrophs are stimulated (to release FSH and LH) and inhibited by GnRH and gonad hormones, respectively.
[edit] Hypothalamus and Pituitary anatomy
- An illustration highlighting the point that gonadotropes reside in the anterior pituitary.

[edit] Pulsatile versus continuous GnRH
- When you override the pulsatile release of GnRH by infusing lots continuously, LH / FSH drops to low levels.
- So we can see that it is important that GnRH must be released pulsatile to get normal release of LH / FSH.
[edit] Control of the onset of puberty
- Puberty: the period of transition between juvenile state and adulthood, during which secondary sex characteristics appear and fertility is acquired.
- We say that puberty occurs when the HPG axis matures, but we don't know the catalyst for puberty.
- We do know that the onset of puberty is affected by many factors, including: genetics, nutrition, body weight, skeleton maturation (affects estrogen levels), altitude.
- We suspect that psychosocial and environmental factors (like environmental estrogen exposure) also play a role in determining the onset of puberty.
[edit] Mini-puberty of infancy in males
- In males, during the first month of life, there is a period of adult-like HPG axis activation.
- That is, a period where testosterone levels are equal to those of adult males.
- The function of this mini-puberty in boys is unknown.
- There is no appreciable change in physical characteristics caused by these high levels of testosterone.
[edit] Testosterone throughout the lifespan
- Testosterone is seen during the first and second trimesters of pregnancy, primarily.
- Then test is expresed during the mini puberty and begins to ramp up again during the 10-17 years (puberty).
- Test expression remains constant through most of adult life and then begins to fade in old age.
- http://books.google.com/books?id=9gvBlktAT6YC&lpg=PA1&ots=L23cN_r6NM&dq=kaefer%20m%20Mechanisms%20manifestations%20and%20management&lr&pg=PA256#v=onepage&q=testosterone&f=false
[edit] Changes in the HPG axis during puberty
- During puberty, the HPG axis is "maturing".
- Decreased sensitivity of GnRH-releasing neurons (hypothalamus) to negative feedback (from the gonad hormones) causes an increase in pulsatile GnRH release.
- Increased sensitivity of gonadotrophs (anterior pit) to GnRH causes an increase in LH / FSH secretion.
- Increased sensitivity of gonads to LH / FSH causes increased gonadal steroid production.
- stopped here on 04/04/11.
- started here on 04/05/11.
[edit] Kisspeptin and GPR54 at the Hypothalamus
- Neurons of the hypothalamus is stimulated to release GnRH when kisspeptin binds GPR54.
- GPR54 is a 7-transmembrane protein: bind extracellular signal and then transduce the signal via the cytoplasmic tail.
- Kisspeptin is one of several peptides encoded by the Kiss-1 gene.
- When GPR54-Kisspeptin signaling is interrupted, hypogonadotropic hypogonadism results from reduced LH / FSH signaling.
[edit] Characteristics of normal puberty
- There are four aspects to a normal puberty phase.
- Sexondary sexual characteristics develop: things that are not directly related to making babies (facial hair, breast enlargement, et cetera).
- Somatic growth spurt occurs
- Fertility is acquired
- Physiological changes occur
[edit] Puberty terminology
- Adrenarche: onset of adrenal and androgen production
- Precedes puberty by 2-3 years
- Occurs around 7-8 years old
- Thelarche: onset of breast bud development
- Estrogen causes thelarche
- Greek / latin: thel- nipple, female
- Pubarche: onset of pubic hair growth
- Estrogen or testosterone causes pubarche.
- Menarche: onset of menstral flow
- Average age of menarche onset in the US is 12.8 years old
[edit] Secondary sexual development
- Gonadarche: rise in gonadal sex steroids as a result of the HPG axis re-activation (recall that it was active in pre-natal development).
- Adrenarche: rise in adrenal androgens independent of gonadal sex steroid production
- We know that estrogens and androgens cause some of the changes seen in puberty because aberrant exposure to estrogens and androgens causes aberrant changes.
[edit] Physical effects of sex steroids
- Estrogena 'and androgens cause growth acceleration, skeletal maturation, and genital changes.
- Estrogens cause breast development in both boys and girls.
- Androgens cause body hair, body odor, and also causes acne in both boys and girls.
[edit] Puberty in girls
- Age of onset between 7.5 years to 13 years; average age of onset is 10.25.
- The first sign of puberty is breast buds in 70% of cases.
- Another common first sign is pubic hair.
- A second sign of puberty usually follows within 6 months.
- The peak growing time for women usually occurs 1.3 years before menarche.
- Average growth during this growth period is 9 inches.
- Menarche usually occurs 2.25 years after the onset of puberty.
[edit] Puberty in boys
- Age of onset between 9 years to 14 years; average age of onset is 12.25.
- The first sign of puberty in boys is testicular enlargement.
- One can measure the testicular volume as an indicator of enlargement.
- The peak growing time for men is usually 2 years later than in girls.
- Boys usually gain around 11 inches during pubertal growth spurt.
[edit] Puberty comparison: boys and girls
- Boys start and end later.
- Girls start earlier and proceed more rapidly through puberty.

[edit] Abnormal puberty
- There are lots of causes of abnormal puberty--some are normal variation and some are pathological.
- Any junction of the HPG axis can be involved.
- The treatment depends on the etiology.
[edit] Precocious puberty
- Precocious puberty defined as "secondary sexual development occurring in girls before the age of 7.5 / 8 (AA, Hispanic / caucasians) or in boys before the age of 9".
- There are 3 types of precocious puberty: normal variants, central, and peripheral.
- Normal variants resulting in precocious puberty can occur by way of premature thelarch (recall that thel refers to breast in greek or latin) or premature adrenarche (adrenal or adrogen production).
- Central precocious puberty arises from defects of the HPG axis.
- Peripheral precocious puberty arises from an ectopic (non HPG) source of sex steroids.
[edit] Central precocious puberty
- Most cases of precocious puberty are central precocious puberty (having to do with the HPG axis).
- Central pp (precocious puberty) results in a normal sequence of events just at an earlier time; that is, it looks just like puberty but occurs earlier in the patient's life.
- Central pp is much more common in females.
- Central pp: females > males
- Central pp's etiology is usually idiopathic.
- CNS injuries can increase the risk for central pp. (Blows to the head, spinal injuries, etc.)
- Secondary sexual development occurs gradually.
- Somatic growth (which is a normal part of puberty) also starts early, is accelerated, and is then arrested relatively early (even for precocious puberty) and thus results in short stature.
[edit] Causes of precocious puberty
- Recall that precocious puberty is ultimately the early release of sex hormones.
- Tumors or hyperactivity of the pituitary or hypothalamus can cause early release of the sex hormones.
- 60% of pp boys have an identified brain abnormality.
- Most girls under 4 with pp have an identified brain abnormality.
- 80% of girls with pp do not have an identified brain abnormality.
- Pseudoprecocious puberty results from a tumor of the adrenal / testes / ovary that releases sex hormones.
- In pseudoprecocious puberty, the gonads do not develop early (because they are not getting the required LH / FSH signaling) but the aberrant levels of sex hormones will cause secondary sexual development.
[edit] Precocious puberty: Symptoms and diagnosis
- Male and female S&S: underarm / pubic hair growth, body odor change, acne, early growth, early arrest of growth, short stature,
- Male S&S: facial hair growth, penis lengthening, appearance becomes masculine
- Female S&S: menstruation, breast development
- Recall that one difference between true and pseudo- precocious puberty is the development or lack of development in the gonads, respectively.
- In true precocious puberty, the gonads develop because there are elevated levels of LH and FSH.
- In pseudoprecocious puberty, the gonads do not develop because there are not elevated levels of LH and FSH.
- Diagnostics include measuring blood hormone levels and taking x-rays of the hand and wrists for estimates of bone development.
- CT, MRI, and ultrasound are also used to look for adrenal / hypothalamic / pituitary tumors and development of the adrenals and gonads.
[edit] A GPR54-activating mutation
- Recall that the GPR54 receptor resides on the neurons of the hypothalamus (in the MBN) and is activated by kisspeptin.
- This research identified a mutation in the GPR54 receptor that activated the receptor and caused central precociouis puberty.
- Recall that turning on GPR54 increases GnRH which increases LH / FSH at the pit which causes development of the gonads.
- Specifically, the mutation caused a decrease in receptor desensitization such that the receptor transduced an intracellular signal for a longer period of time than a wild-type receptor.
- This decreased densensitization caused increased signaling through the GnRH releasing neurons and increases GnRH release.
- This image shows the amount of phosphorylated ERK as a measure of pathway activation.
- In the disease state, there is more phosphorylated (activated) ERK present.
[edit] Peripheral precocious puberty
- Recall that peripheral precocious puberty occurs when sexual development is induced by sex steroids that do not originate from the HPG axis.
- Peripheral precocious puberty is rare and can be heritable or not.
- The non-HPG source of steroids can be endogenous or exogenous.
- Peripheral precocious puberty often demonstrates heterogeneity:
- there is often acute onset,
- there is often linear growth acceleration that results in tall stature and advanced bone age (upon xray diagnostics on the hand and wrist),
- there are many different classes of steroids to which children can be exposed,
- the duration of exposure to steroids can be quite variable.
[edit] McCune-Albright syndrome, a form of peripheral precocious puberty
- One cause of peripheral precocious puberty has been named: McCune-Albright syndrome results from an activating mutation of a G protein expressed in endocrine tissues.
- The G protein's Gs-alpha subunit is mutated into a higher activity state causing increased cAMP.
- Elevated cAMP from an over-active G protein causes hyperfunction of endocrine tissues.
- McCune-Albright is characterized by a triad of symptoms: pp, cafe au lait, and fibrous bone dysplasia.
- Large ovarian cysts are also seen in girls.
- McCune-Albright precocious puberty is an example of a somatic mutation in a mosaic distribution.
Why is it mosaic? Because it mutates during development? Yes per wikipedia
[edit] Delayed puberty
- We consider puberty delayed if there is no female onset by 13 or male onset by 14.
- We also consider pubertal development slower than one Tanner stage per year delayed puberty.
- Delayed puberty can either be "normal variant" or pathologic.
- Normal variant delayed puberty shows similar delay in both somatic growth and sexual development and often occurs with a family history of "late bloomers".
- Pathologic delayed puberty can be congenital or acquired and may be caused by a problem at any level in the HPG axis.
[edit] Conclusion
- The HPG axis is a highly integrated system with inhibitory and stimulatory modulators.
- Though we don't know the trigger for puberty, we do know the predictable series of events that normally occur.
- There are many different etiologies for abnormal puberty, many of which affect the HPG axis.
- stopped here on 04/05/11.
- started here on 04/06/11.
[edit] Male reproductive physiology
[edit] Learning objectives
- Recognize the structure and functions of the male reproduction organs
- Understand the process of spermatogenesis
- Understand steroidogenesis
- Understand disorders of male reproduction
[edit] Anatomy
- The creamaster muscle and the pampiniform plexus help regulate the temperature of the testes.
- The cremasteric muscle can pull the testes up toward the abdomen for increased heat.
- The pampiniform plexus of vessels provides lots of blood flow and lots of heat.
How much more anatomy do I need to know from this slide?
[edit] Characteristics of the testes
- The testes have two functions: spermatogenesis and steroidogenesis.
- The seminiferous tubules are the site of spermatogenesis.
- The seminiferous tubules are very small tubules that radiate through the testicle from the rete testis.
- Two cell populations comprise the seminiferous tubule: germ cells and Sertoli cells.
- Germ cells generate new sperm.
- Sertoli cells play a supporting role to germ cells.
- Leydig cells produce testosterone.
- Leydig cells are also called "interstitial cells of the testes" because they reside between the seminiferous tubules.

- Note the epididymis, seminiferous tubules, lobules, and vas deferens.
[edit] Structural organization of the testes
- The cellular organization within the seminiferous tubules is focused on generating a blood-testes barrier.
- The blood-testes barrier is important to prevent immunological attack upon the developing sperm.
- To form this barrier, sertoli cell surround the germ cells that generate sperm.
- Sertoli cells span the entire width of the seminiferous tubule.
- There are two compartments in the seminiferous tubules: basal and adluminal compartments.
- The basal compartment contains the least mature sperm and is nearest the border of the tubule.
- The adluminal compartment contains the most mature sperm and is in the center of the tubule.


[edit] Sertoli cell supportive functions
- Recall that sertoli cells are supportive cells to germ cells.
- Sertoli cells are important for spermatogenesis in three ways: phagocytizing defunct germ cells, spermiation, synthesis of transferrin.
- Spermiation: "the release of the spermatozoon from the seminal epithelium into the lumen of the seminiferous tubule." per academia
- Sertoli cells provide plasmin, an enzyme that causes release of spermatozoa from the epithelium of the seminiferous tubule into the lumen.
- Recall that spermatozoa are fully mature male gametes.
- Note that transferring is important for spermatogenesis.
[edit] Sertoli cell secretory functions
- Sertoli cells have g-protein FSH receptors that drive cAMP and PKA.
- cAMP and PKA in sertoli cells have several effects, all of which generally lead to increased signaling and proliferation.
- Increased release of androgen binding protein.
- Recall that ABP carries testosterone and DHT in the blood.
- Increased release of inhibin.
- Recall that inhibin inhibits the pituitary (FSH / LH).
- Induction of P450 aromatase
- Recall that P450 aromatase converts testosterone to estradiol.
- Increased release of plasminogen activator
- Recall that plasminogen activator is a serine protease that breaks down clots.
- Increased cell proliferation
- Increased release of androgen binding protein.
[edit] Sertoli cell products
- Sertoli cells proudce three major products: ABP, Inhibin, Plasminogen activator
- Androgen binding protein:
- 90Kd, heavy and light chain
- Binds Test and DHT with high affinity
- Used for carrying androgens in the Sertoli cells and epididymis.
- Used for storing androgens in the seminiferous tubule
- Inhibit
- Provides negative feedback to the pit.
- Plasminogen activator
- Cuts plasminogen into plasmin which goes on to digest fibrin (a key structural protein in clotting).
[edit] Leydig cells
- Recall that leydig cells reside in the interstitium, between seminiferous tubules.
- Know this, it kind of makes sense that leydig cells are derived from mesenchymal cells as they are sitting in a connective tissue area.
- Leydig cells are especially focused around blood vessels.
- Leydig cells are primarily a reservoir of lipids that can be used to generate testosterone.
- Leydig cells appear foamy because of the presence of secondary lipid droplets.
- The lipid droplets are esterified cholesterols which can be used for testosterone production.
- Testosterone production requires two major steps and three locations:
- The first major step occurs in the cytoplasm: hydrolyzing the esterified cholesterol into free cholesterol.
- The second major step occurs in the mitochondria: converting cholesterol into pregnenolone.
- Note that chol->pregnenolone is the rate-limiting step.
- The third location is the endoplasmic reticulum where testosterone is finally generated.
[edit] Leydig actions
- Leydig cells have LH receptors that stimulate release of testosterone.
- Sertoli cells and Leydig cells demonstrate reciprocal hormonal communication.
- As the Leydig cells release testosterone (via LH signaling), the Sertoli cells use their P450 aromatase to convert it to estradiole (E2) and send it back to the Leydig cells.
- The function of E2 (estradiol) signaling on Leydig cells is unclear.
[edit] Additional products of the testes
- The testes also make several other products:
- Opiods
- AVP
- Oxytocin
- GnRH-like peptide
- Growth factors
- Neurotransmitters
Anything more to know from this slide?
[edit] Spermatogenesis
- Spermatogenesis is a well scheduled event so there are phases and cycles.
- The three phases are: mitosis, meiosis, and spermiogenesis
- Cycles last 65-70 days and new cycles begin every 2-3 weeks.
[edit] Germ cell mitosis and meiosis
- The cells of spermatogenesis proceed in a particularly named order through a series of specific types of divisions:
- Cell (count): primordial germ cell (1) -> spermatogonia (1) -> primary spermatocyte (32) -> secondary spermatocyte (64) -> spermatids (64) -> spermatozoa (64)
- Note that primordial germ cells, spermatogonia, and primary spermatocytes are diploid whereas secondary spermatocytes, spermatids, and spermatozoa are haploid.
- Divisions: Mitosis -> meiosis 1 -> meiosis 2.



[edit] Spermiogenesis
- Spermiogenesis (as opposed to spermatogenesis) is the specialization of the spermatid into the spermatozoa.
- Four major changes take place to form a highly specialized cell:
- Nearly all the cytoplasm is lost.
- Nuclear chromatin is condensed and altered.
- The axoneme (tail) is formed: centrioles rearrange and relocated to form a 9x2 arrangement on the cell membrane.
- the axoneme has a fibrous sheath, too.
- The acrosome is foromed: a collection of enzymes surrounds the nucleus at the opposite pole as the tail.
- There are three major sections to the completed spermatozoa:
- The head, made of the acrosome (collection of enzymes).
- The middle, made of spiral sheathes of mitochondria for energy.
- The tail--also called the axoneme--propels the cell forward by a twisting motion.
[edit] The axoneme
- Recall that the axoneme is generated from the spermatid's centriole.
- There is an intricate structure to the axoneme that is important for its function.
- At the center is the central tubule with 2 tubules.
- Radiating from the outside toward the center are the radial spokes.
- Radial spokes are connected to the exterior structure by Y links.
- Radial spokes are connected to adjacent radial spokes by bridges.
- Along the radial spokes are 9 sets of doublet tubules.
- The axoneme uses dyenein motors on the doublet tubules to generate a spiral twisting which generates movement.
[edit] Role of testosterone and FSH in spermatogenesis
- Both FSH and testosterone are required for spermatogenesis.
- Recall that Sertoli cells have receptors for both FSH and testosterone.
- Recall that testosterone is generally coming from the Leydig cells because of LH signaling.
- Recall that FSH is coming from the anterior pituitary.
- While FSH is required for initiation of spermatogenesis, testosterone is sufficient to maintain progressing spermatogenesis.
- Testosterone is required for capacitation: the process that allows spermatozoa to generate motion and occurs in the epididymis.
[edit] Seminal fluid
- Seminal fluid (semen) is the entire combination of fluids ejaculated.
- Seminal fluid contains:
- 10% sperm by volume
- seminal vesicle fluid: 75% by volume
- prostatic secretions
- bulbourethral secretions
- Seminal fluid has fructose (nutrient for spermatozoa), ascorbic acid (I think has something to do with maintaining pH once in the female UG tract), prostaglandins (increase blood flow), and fibrinolysin (cut up clots).
[edit] Expulsion of semen
- Semen expulsion is a neuromuscular reflex with two phases: emission and ejaculation.
- Emission moves sperm and the rest of the seminal fluid contributions into the urethra.
- The lumbar spine nerves control the muscular contractions of the epididymis and vas deferens.
- Ejaculation moves semen out of the urethra.
- Ejaculation requires a second spinal reflex and contraction of the bulbospongiosus muscles that surround the uretra.
- Recall from anatomy that Pointing and Shooting require Parasympathetics and Sympathetics, respectively.
[edit] Testicular steroidogenesis
- Recall the hormone synthesis pathway of the adrenal gland:
- Which of these end products is produced is determined by the predominant enzyme present.
- By genetic determination of the predominant enzyme expressed, tissues like the testes, the adrenal glands, and the ovaries can become functionally specific.
- In the testes, testosterone is the primary product generated.
- Pregnenolone is the key intermediate for the testes.
- Note that delta 5 or delta 4 can be used along the pregnenolone -> testosterone pathway.
[edit] The LH receptor
- Recall that the Leydig cells contain cholesterol stores and generate testosterone upon LH signaling.
- Leydig cells have 15K LH receptors / cell yet less than 5% need to be activated to generate a response.
- This means that Leydig cells can detect very low levels of LH.
- The LH receptor signals through a g-protein coupled receptor, cAMP, and PKA.
- Ultimately, testosterone is generated and released.
[edit] Familial male precocious puberty (FMPP)
- Familial male precocious puberty (FMPP) is also called testotoxicosis.
- Testotoxicosis is "familial" because a mutation of the LH receptor can cause this disease.
- Mutations of the LH receptor have been shown to cause excessive inactivation (and thus excessive and early release of testosterone and thus precocious puberty).
- At least 15 mutations have been identified.
- Most mutations involve ASP478.
- Clinical manifestations of FMPP include:
- precocious puberty by age 2-6
- secondary sexual development
- acne
- growth acceleration and advanced skeletal maturation
- behavior problems
- Note that female carriers of the mutated LH receptor are unaffected.
- Treatment of FMPP:
- Apply an androgen receptor blocker to inhibit the excessive and early testosterone.
- Apply an aromatase inhibitor lest the testosterones get aromatized into estrogen (via Sertoli cells as one example).
[edit] Metabolism of testosterone
- Recall that testosterone is generated by Leydig cells upon LH signaling.
- Testosterone is a steroid so it can immediately diffuse out of the producing cell into the blood.
- In the blood, most testosterone (97-98%) is bound to carrier proteins (steroid hormone binding globulin, SHBG).
- SHBG acts as a reservoir for testosterone.
- SHBG is increased by estradiol signaling on the liver.
- Plasma testosterone is generally converted to DHT or E2 (estradiol) at the target tissue:
- DHT: prostate, scrotum, penis, bone
- E2: fat, liver, CNS, skin, hair
- 17 ketosteroid: liver, kidney
- Test: testes, pituitary, muscle
- Conjugate: liver, kidney
[edit] DHT, dihydrotestosterone
- DHT is two to three times more potent than testosterone.
- DHT is critical for normal sexual differentiation.
- 5-alpha reductase converts testosterone to dihydrotestosterone (DHT).
- 5-alpha reductase is also active in hair follicles and sebaceous glands.
[edit] Estradiol
- Estradiol is generated by many tissues (including testes and the brain) from testosterone.
- Abnormal levels of E2 causes gynecomastia (breast enlargement).
*E2 levels in men rise nearly to those of women when the man goes through the follicular phase. Does he really mean this?
[edit] Actions of androgens
- Androgens have affects on physical differentiation, brain masculinization, and physiological properties.
- Physical differentiation: sexual differentiation (see Sexual differentiation, HPG axis), secondary sexual characteristic development (hair, acne, voice, growth), hair growth (and balding).
- Physiological properties: lipid levels, RBC mass
- Masculinization of the brain
- There are many androgen receptors on the brain.
- Although we know that hierarchy and copulating patterns in animals are correlated with androgens, there is no correlation between libido and androgen levels in human males.
- Females exposed to excess androgens during development (in utero) demonstrate increased "male typical" behavior.
[edit] Worldwide trends in male reproductive function
- We are currently observing trends that make us think reproductive function may be declining:
- serum testosterone levels are decreasing in the US and Europe.
- Sperm concentration has decreased by nearly half over the past 70 years.
- Semen quality differs geographically and has been shown to correlate with pesticide expsoure (less semen production upon exposure).
- There is an increased incidence of cryptorchidism, hypospadias, and testicular cancer.
- Cryptorchidism: "a condition seen in newborns whereby one or both of the male testes has not passed down into the scrotal sac." per Driscoll Chidlrens
- Hypospadias: "an abnormal condition in males in which the urethra opens on the under surface of the penis" per Princeton's wordnetweb
[edit] Disorders of male reproduction
- Half of the infertility of men is caused by endocrine disorders.
- Hypothalamic-pituitary hypogonadism results from a defect in the HPG axis: Kallman syndrome, GPR54 gene mutations, and GnRH receptor mutations.
- Recall that GPR54 receptor mutations lead to decreased GnRH release by the neurons of the hypothalamus upon kisspeptin signaling.
- Recall that GnRH receptor mutations lead to decreased LH / FSH release by the gonadotrophs in the anterior pituitary upon GnRH signaling from the hypothalamus.
- 'Primary hypogonadism occurs because of defects in the gonads: Klinefelter syndrome, testicular regression, acquired hypogonadism
- Recall that Klinefelter syndrome results from a 47XXY karyotype that causes underdeveloped testes and therefore decreased testosterone levels.
- Note that testicular regression syndrome covers "a variety of conditions in which both testes regress during fetal life. It is also known as pure gonadal dysgenesis, Sawyer's syndrome, true agonadism, testicular dysgenesis, vanishing testis and complete bilateral anorchia" per Medcyclopaedia.
- Recall that "true" gonadal dysgenesis resulted from a defect in the SRY region.
- Acquired hypogonadism can occur because of infection or abuse of anabolic steroids.
- Other endocrinopathies: hyperprolactinemia, excess androgens
[edit] Kallman syndrome
- Kallman syndrome results from the mutation of the cell adhesion gene KAL.
- KAL is ubiquitously expressed in all tissues of the body and even escapes X inactivation.
- KAL is located on the p arm of the X chromosome.
- The KAL gene generates anosmin as a protein product; anosmin is an important component of the basement membrane.
- When KAL is mutated in XY males, hypogonadotropic hypogonadims and anosmia results.
- Without functiona anosmin, GnRH secreting neurons of the olfactory placode fail to migrate to the medial basal hypothalamus (MBH).
- Improper migration leads to olfactory bulb dysgenesis and decreased GnRH release:
- In some cases, the pt cannot smell
- In most cases, the pt has poor gonad development, poor secondary sexual characteristic development, and poor puberty development.
[edit] Conclusions
- Testes produce both steroids and sperm.
- Spermatozoa are the end product of spermatogenesis and are highly specialized cells.
- Androgens affect physical appearance, the brain, and physiology.
- Disorders of male reproduction can result from endocrine and non-endocrine (Kallman Syndrome) etiologies.
- stopped here on 04/07/11.
- started here on 04/07/11.
[edit] Female reproductive physiology
[edit] Objectives
- Know key characteristics of the H-P-O Axis
- Recognize the structure and functions of female reproductive organs
- Understand Oocyte Mitosis and Meiosis
- Understand Folliculogenesis, Steroidogenesis, Ovulation
- Know key aspects of the menstrual cycle
- Recognize abnormalities of female reproduction
[edit] HPO axis
- The HPO axis is the hypothalamus-pituitary-ovary axis.
- Recall that the hypothalamus releases GnRH which stimulates gonadotropes of the anterior pituitary to release LH / FSH which stimulate the ovaries to secrete estrogen and progesterone.
- Pituitary release of LH and FSH can be tonic (consistent at one level) or come in surges.
- Hormone release along the HPO axis is cyclic and highly synchronized.
- Feedback mechanisms of the female HPO axis differ from those of the HPT axis of men.
- While androgens negatively feed back on the hypothalamus, progesterone and estrogen can positively or negatively feed back on hypothalamic GnRH release depending on the amount produced.
- Also, unlike the LH / FSH negative feedback on GnRH release in males, in females, LH / FSH do not negatively feed back on GnRH release at the hypothalamus.
[edit] Characteristics of female fertility
- Female fertility has several dogmatic characteristics:
- There is a finite period of fertility in women, namely menarche to menopause.
- Reproductive capacity is intermittent meaning that there are cycles in which reproduction can and cannot occur.
- Note that the reproductive cycle is easily disrupted.
- Germ cells decrease over time; that is, germ cells are not continuously generated as in males and thus decrease in number over time.
- One ova is released at a time.
[edit] Determinants of menarche onset
- Menarche is the beginning of the ovulatory cycle and marks the beginning of fertility.
- The commencement of menarche is determined by genetic, nutritional, and environmental factors.
- Timing of menarche have been shown to be strongly correlated among familes.
- Obesity has been shown to accelerate the onset of menarche.
- Family composition and history of abuse have been shown to affect menarche.
***More females in the family is associated with earlier menarche. ***Abuse induces earlier menarche.
[edit] Female reproductive organs
- There are two major compartments of female reproductive organs: the ovaries and the ductal system.
- The ovaries are responsible for germ cell maturation and steroidogenesis.
- The ducts are responsible for transporting the ova (unfertilized female gamete) and maintaining the conceptus throughout the pregnancy.
- Ova = ovum: "ovum - the female reproductive cell; the female gamete" per Princeton's wordnetweb
- Conceptus: "embryo: an animal organism in the early stages of growth and differentiation that in higher forms merge into fetal stages but in lower forms terminate in commencement of larval life" per Princeton's wordnetweb
[edit] Ovarian structure
- The ovaries are the germ cell incubators in the female GU tract.
- Much like the testes, the ovaries are encapsulated by the tunica albuginea (alb: white).
- The ovary has two compartments: inner and outer medulla.
- The outer medulla contains multiple follicles at subsequent stages of development.
- Ovar reside within the follicles.
- The inner medulla contains interstitial and connective tissues.
[edit] Ovarian anatomy
- Recall that the ovary is attached to the posteriolateral wall of the abdomen by the ovarian ligament.
- Through the ovarian ligament travel the ovarian blood vessels to supply the ovaries.
- Recall that the parenchyma and the stroma are opposites: functional tissue of the organ versus structural tissue of the organ.
- Stroma: "the supporting tissue of an organ (as opposed to parenchyma)" per Princeton's wordnetweb
- Note that stroma cells produce androgens.
- The ovary ovulates (releases a mature ova) near the fimbria of the ovarian duct.
- The ovarian duct is made up of the ampulla and the isthmus.
- The isthmus of the ovarian duct receives the ova from the ampulla and shuttle it through the uterine wall to corpus of the uterus.
- Recall that the uterus is composed of the fundus, the corpus (body), and the cervix (neck).
- Recall that the uterus is held in place by the broad ligament of the abdomen.
- The uterus is composed of two layers: the endometrium and the myometrium; the myometrium provides the muscle of contraction.
- The ductal system to carry the ova continues through the corpus of the uterus, through the internal and external os, through the cervix (passed the two fornices), and through the vagina.
[edit] Primary hormones produced by the ovary
- Recall that the ovary is responsible for germ cell maturation and steroidogenesis.
- The ovary produces estrogen, progesterone, inhibin, and activin.
- Estrogen is made by the follicles.
- Follicles contain the ova and mature / degenerate as they travel up and down the ovary.
- Progesterone is produced by the corpus luteum.
- The corpus luteum is the degenerating follicle.
- Inhibin negatively feeds back on the anterior pituitary (and not the hypothalamus).
- Estrogen is made by the follicles.
[edit] Ovarian steroidogenesis
- The primary hormonal product of the ovaries is estradiol.
- The production of hormones in the ovary requires two cell types and two hormonal signals:
- Theca cells are signaled by LH to produce testosterone.
- Granulosa cells are signaled by FSH to convert testosterone into estradiol (E2) via aromatase.
- Recall that aromatase converts testosterone to E2.
- Granulosa cells:
- Granulosa cells are the only cells of the ovary to express the FSH receptor.
- FSH can induce expression of its own receptor!
- Granulosa cells lack Cyp17, so they cannot generate androgens on their own.
- Recall that Cyp17 is required to convert cholesterol to androgens.
- That is granulosa cells depend on theca cells as a source of androgens.
- Granulosa cells do begin to express the LH receptor as the follicle matures.
- This makes sense as it will undergo luteinization after ovulation.
- Theca cells:
- Recall that theca cells are signaled via LH to produce testosterone.
- Theca cells use cholesterol from the blood and Cyp17 to provide testosterone to the granulosa cells.
- Recall that theca cell are on the outside of the follicle--nice, easy access to blood and cholesterol.
- Theca cells produce primarily androstendione.
- Androgens have pro-aromatase activity at low levels and pro-atresia activity at high levels.
- Stroma cells:
- Stroma cells of the follicle are primarily structural connective tissue.
- However, stromal cells can produce some androgens.
[edit] Oocyte development
- As with spermatogenesis, a germ cell precursor yields a series of intermediate cells and finally a mature gamete.
- Recall that the testes are the home of germ cell maturation in males; similarly, the ovary is the site of germ cell maturation in females.
- Also as with males, the germ cell uses a series of mitosis and meiosis to generate mature gametes.
- Cells: oogonia -> primary oocyte -> secondary oocyte (ovum).
- Divisions: mitosis -> meiosis 1 (arrested in phrophase of meiosis 1) -> meiosis 2 (arrested in metaphase of meiosis 2).
- From the top:
- Oogonia (primordial germ cells) originate at the yolk sac and migrate to the genital ridges.
- Note that this step would be the same in males but the genital ridges would become testes, not ovaries.
- Oogonia undergo mitosis to increase in number during the first two trimesters.
- Oogonia mature into primary oocytes; note that this maturation process does not generate new cells.
- Primary oocytes arrest in prophase of meiosis 1.
- Recall that in prophase of meiosis 1, the primary oocyte has made a copy of the genome (and so is 4N), sister chromatids are attached by their centromeres, homologous chromosomes have crossed over, and the centrosomes are moved to the poles.
- Primary oocytes remain in this state until the onset of regular menstruation cycles.
- Note that this is a long period of time during which oocytes are exposed to toxins which can result in chromosomal defects.
- Upon LH spike, primary oocytes progress to secondary oocytes.
- Completing the first division of meiosis (that is, completing meiosis 1, a mitotic like event) generates the first polar body.
- LH spices during menarche and at each subsequent ovarian cycle (period).
- Secondary oocytes are arrested in the metaphase of meiosis 2.
- Recall that in metaphase of meiosis 2, the secondary oocyte has gone through one division (and so is 2N), sister chromatids are attached by their centromeres, and centrosomes have attached spindles to the kinetochores.
- Metaphase of meiosis 2 is also called the ovulatory stage.
- Secondary oocytes remain in metaphase of meiosis 2 until sperm penetrate the cell.
- Sperm penetrate the secondary oocyte, inducing completion of meiosis.
- Completion of the second cell division (that is, completing meiosis 2) generates the 'second polar body.
- Oogonia (primordial germ cells) originate at the yolk sac and migrate to the genital ridges.
- Oocyte count along lifetime:
- Week 20 of gestation: 6 million
- Week 40 of gestation: 2 million
- Menarche: 500K
- Menopaue: 0
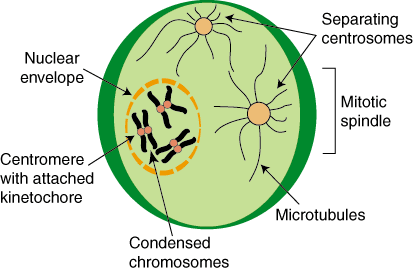
[edit] The mature follicle
- The mature follicle has 7 layers and 1 space:
- The layers from outside to inside: theca externa, theca interna, basement membrane, granulosa cells, corona radiata (around most of the oocyte), cumulus oophorus (connecting the oocyte with the granulosa cell layer), and the zona pellucida.
- The antrum is a space filled with follicular fluid between the granulosa cells and the corona radiata layer.

- Theca interna / externa.
- The thecal cells of the theca externa and theca interna are signaled by LH to produce androgens that affect the granulosa layer.
- Theca cells have ready access to choleterol from the blood stream (they are on the outside where it is easier to get stuff from the blood).
- Theca cells primarily produce androstendione.
- Androgens have different effects at different levels: high -> follicular atresia, low -> increased aromatase (testosterone -> E2) activity.
- Basement membrane
- The basement membrane holds the follicle together until just before ovulation when the basement membrane disintegrates.
- Granulosa
- Granulosa cells of the granulosa layer are responsible for generating follicular fluid and converting androgens to E2.
- Granulosa cells have an FSH receptor through which they are siganled to convert androgens to E2 (via aromatase).
- Recall that theca cells generate androgens upon LH signaling; it is from theca cells that granulosa cells receive their supply of androgens.
- FSH induces expression of its own receptor in the granulosa cells.
- As the follicle matures, FSH and E2 induce expression of the LH receptor in granulosa cells.
- It makes sense that the LH receptor should begin to be expressed in the granulosa cell because just before ovulation, the granulosa cells will leutinize to become vascular, lipid-rich luteal cells.
- Antrum
- The antrum is filled with follicular fluid produced by granulosa cells.
- Follicular fluid contains FSH, hormones, growth factors, opioids peptides, and plasminogen activators.
[edit] Folliculogenesis
- Follicle progression follows a series of maturation (folliculogenesis) steps up one side of the ovary and then goes through ovulation (releases a mature ova).
- Maturation: primordial follicle -> primary follicle -> secondary follicle -> tertiary follicle -> Graafian follicle.
- There are particular characteristics associated with each follicle state:
- primordial follicles have a primary oocyte and a single layer of granular cells.
- primary follicles have a larger oocyte, the first appearance of the zona pellucida, and a layer of granular cells.
- secondary follicles have an oocyte, zona pellucida, multiple layers of granulosa cells, and the first appearance of theca cells.
- tertiary follicles have an oocyte, zona pellucida, multiple layers of granulosa cells, appearance of the antrum, and distinction of the theca interna and externa.
- Graafian follicles have an oocyte, zona pellucida, appearance of the cumulus oophorus, appearance of the corona radiata, multiple layers of granulosa cells, antrum, theca interna, and theca externa.

[edit] Role of FSH in folliculogenesis
- Recall that granulosa cells have FSH receptors and are a significant layer of the follicle.
- Recall that the granulosa cells have serve to produce E2 and follicular fluid.
- Recall that follicular fluid is rich in FSH.
- FSH signaling on the granulosa cells causes:
- Increased E2 production (from androgens from theca cells),
- Granulosa proliferation.
- Granulosa proliferation increases the follicle's capacity to produce E2, to generate fluid, and to bind FSH.
[edit] Regulation of folliculogenesis
- Recall that the primordial follicle first progresses to the primary follicle stage.
- Movement from the primary follicle to the primary follicle is gonadotroprin-independent.
- That is, consistently throughout life and without the need for gonadotropins primordial follicles are progressing to primary follicles.
Even before puberty?
- Progression from a primary follicle to secondary follicle and beyond is gonadotropin-dependent.
- In most cases, only one follicle per ovarian cycle will progress to secondary follicular stage.
- Note that follicles that progress past the primary follicle stage are committed.
[edit] Ovulation
- During ovulation, one of the developing follicles becomes predominant and will rapidly mature and eject its ova to the fimbria.
- The general steps in ovulation include establishing the dominant follicle, atresia of other follicles, LH spike to prepare the microenvironment, acute pre-ovulation changes, and ejection of the ova.
- The dominant follicle is usually established by 5-7 days into the ovarian cycle and will undergo exponential growth.
- As the dominant follicle grows, granulosa cells proliferate and consequently follicular androgen and E2 levels rise.
- As E2 levels rise there is increased feedback on the pituitary, decreased FSH, and therefore decreased gonadotropic effect to help the non-dominant follicles develop.
- Because of these decreased levels of FSH, the non-dominant follicles undergo atresia.
- Non-dominant follicles are generally pre-antrum (that is, secondary follicles or even less mature).
- Upon LH spike, the dominant follicle undergoes a series of changes that prepares it for release of the ova and prepares the microenvironment for fertilization:
- Meiosis reinitiates (moving form prophase of meiosis 1 to metaphase of meiosis 2).
- Steroid production switches from E2 to progesterone.
- This change to production of progesterone is associated with an increase in basal body temperature.
- Blood flow is increased.
What is the scope of this statement? Follicle blood flow, ovarian, uterine?
- Proteolytic enzymes are activated in the follicle.
- 30-36 hours after LH spike the follicle ruptures and ovulation occurs.
- Just before ovulation, the follicle undergoes a series of changes that lead to ovulation:
- Disintegration of the basement membrane,
- Granulosa cells luteinize to become vascular, lipid-rich cells,
- Plasmin cuts up the follicular wall allowing the follicle to enlarge and bulge out from the ovary surface,
- Finally the oocyte is extruded.
[edit] Signs of ovulation
- Signs of ovulation can be understood by examining the changes in hormone levels throughout the process:
- Recall that LH has spike causing an increase in androgens.
- Elevated androgens cause an increased libido.
- Recall that LH has spike causing an increase in androgens.
What about the fact that libido has shown to not be associated with androgens in men?
- Recall that increased androgens get converted to an increase in estradiol.
- Elevated estradiol causes increased spinnbarkeit: "the capacity of a viscous liquid (especially the cervical mucus) to be drawn out into a strand or blown up into a bubble" per Princeton's wordnetweb
- Elevated estradiol causes ferning of cervical mucus: ferning occurs in vivo and helps guide sperm through the cervix per wikipedia
- Recall that steroidogenesis switches from E2 to progesterone.
- Progesterone causes an increase in basal body temperature.
- Recall that increased androgens get converted to an increase in estradiol.

[edit] Follicular fate
- The follicle has two possible fates: atresia or develop into a corpus luteum.
- Most follicles will undergo atresia.
- Corpus luteum formation:
- The corpus luteum is a temporary endocrine structure that develops from a follicle that has evicted its ova.
- Formation of the corpus luteum follows a series of stages like follicular maturity: follicle -> (ovulation) -> early corpus luteum -> mature corpus luteum -> corpus albicans.
- The process of converting a follicle into a corpus luteum is called luteinization.
- Note that lute means "yellow" in Greek and / or Latin.
- So luteinization is a yellowing process.
- In fact, in real life, a fresh corpus luteum is actually yellow.
- The corpus luteum eventually becomes a fibrous structure called the corpus albicans.

[edit] Corpus luteum characteristics
- Recall that the corpus luteum is the post-ovulation continuum of the follicle.
- The corpus luteum is comprised of three cell types: theca cells, stroma cells, and luteal cells (from luteinization of granulosa cells).
- Recall that theca cell generate androgens upon LH signaling.
- Recall that stroma cells can generate some androgens.
- Recall that granulosa cells converted androgens to estrogens and then switched to production of progesterone during ovulation.
- Luteal cells produce both estrogen and progesterone.
- The corpus luteum is the primary source of circulating steroids after ovulation.
- Progesterone levels peak 6-8 days after the LH surge.
- The corpus luteum degenerates after 9-11 days.
[edit] Mentrual cycle
- Menstruation occurs every 28 days, approximately.
- Menstruation occurs as a withdrawal reaction as the hormone levels decrease as the corpus luteum degenerates and stops producing high levels of androgens, estrogens, and progesterone.
- Menstruation can be described as a cycle of changes occurring at two locations: the ovaries (the ovarian cycle) and the uterus (the uterine cycle).
- There are three distinct phases to the ovarian cycle of menstruation: follicular phase, luteal phase, and menses.
- The follicular phase is composed of follicle maturation.
- The follicular phase can be variable in duration.
- During the follicular phase estrogen levels are elevated (recall that androgens are high and granulosa cells are proliferating).
- As estrogen peaks it has a positive feedback effect on the pituitary causing a spike of LH release.
- There is also a small spike of pituitary FSH release.
- LH surge causes a depression of E2.
- Subsequently, the spike of LH causes ovulation.
- The luteal phase begins at ovulation and ends at menses.
- During the luteal phase hormones rise as the corpus luteum matures, then fall as the corpus luteum degrades.
- The luteal phase is consistently 14 days in duration.
- The menses phase consists of shedding the uterine lining.
- During menses, sex hormone levels are low (the corpus luteum has just finished degrading) and FSH is elevated.
- The phases of the menstrual cycle can also be described by the changes to the uterus (the reproductive tract).
- There are four distinct phases to the uterine cycle of menstruation: the proliferative stage, secretory stage, ischemic stage, and menstrual stage.
- The proliferative stage is characterized by endometrium hypertrophy and formation of spiral arteries.
- Recall that the uterine prolierative stage occurs during the ovarian follicular phase.
- So as the ovary is maturing its follicle, the uterus is regenerating it's surface (where the egg will implant) and increasing vascular access to the surface.
- The secretory stage is characterized by coiling of glands, secretion of mucus, tortuous arteries, and peak thickness of the endometrium.
- Recall that the uterine secretory stage occurs during the ovarian luteal phase.
- So, as the ovary has shed an ovum and is now increasing hormone production via the corpus luteum, the uterus is using glands and arteries of the uterus to modify the uterine microenvironment to the optimal conditions for egg implantation.
- The ischemic stage is characterized by arterial constriction, decreased blood flow, and increased prostaglandins.
- Recall that the ischemic stage occurs during the ovarian menses phase.
- So as the ovary has reached its lowest levels of hormone production, the uterus is decreasing nutrition to the endometrium and allowing the mucosa to undergo necrosis by ischemia.
- The menstrual stage is characterized by desquamation of the endometrium.
- Recall that the menstrual stage occurs during the ovarian menses phase.
- So as the ovary has reached its lowest levels of hormone production, the uterus is shedding its endometrium.
- Fortunately, the uterine cycle, the ovarian cycle, and the hormone changes can be summarized with a simple graphic; here are three examples:



[edit] Menopause
- Menopause occurs when no more eggs are available and follicular maturation no longer occurs.
- The average age to run out of eggs (menopause) is 52.
- Menopause is a phenomenon unique to humans.
- Once a patient has run out of eggs, there is less estrogen produced (recall that granulosa cells are the primary source of estrogen and they are found in the developing follicles).
- Less estrogen production means less negative feedback on the anterior pituitary and therefore gonadotropin (LH / FSH) levels are elevated.
- Decreased estrogen also results in hot flashes, decreased bone mass, increased cardiovascular risk, and atrophy of the vaginal epithelium.
- Recall that estrogen inhibits bone mass loss and thus we sometimes supplement at-risk women to prevent osteoporosis.
- Stromal cells of the ovary continue to produce androgens which can be converted to estrone at target tissues.
[edit] Abnormalities of female reproduction
[edit] Precocious puberty in severe primary hyperthyroidism
- Primary hyperthyroidism is an overactivity of the thyroid that can affect the gonads when TSH cross-reacts with the FSH receptor on granulosa cells.
- When TSH levels are aberrantly high (because of a thyroid defect), TSH will signal development of the gonads through the FSH receptor on granulosa cells of the ovaries or the Sertoli cells of the testes.
- TSH-FSHr signaling causes testicular enlargement in boys and breast development and vaginal bleeding in girls.
- It makes sense that TSH-FSHr signaling in girls causes breast development because we know estrogen is what causes breast development and we know that granulosa cells convert androgen to estrogen.
Is this hypothyroidism or hyperthyroidism?
- Patients with primary hypothyroidism will also show the classic signs of hypothyroidism and can be successfully treated with thyroid hormone replacement.
- Recall that S&S of hypothyroidism: Weight gain, Goiter, Puffy appearance, Loss of hair, Low BMR, low body temp, decreased perspiration, Lethargy, depression, intolerance to cold
[edit] Pseudoprecocious puberty versus true precocious puberty
- Recall that pseudoprecocious puberty is gonadotropin-independent; that is, a non-HPG axis source of sex steroids (i.e. androgens or estrogens) has caused secondary sexual development (but will not have caused development of the gonads which require LH and FSH for development).
- Patients with pseudoprecocious puberty show pre-puberty levels of LH but manifest secondary sexual characteristics and early growth arrest.
[edit] Primary amenorrhea
- Primary amenorrhea means that menarche has not occurred.
- Primary amenorrhea can be the result of normal variation, a defect at the hypothalamus or pituitary, a defect at the ovary, or a disorder of sexual development.
- Normal variation in sexual development is considered primary amenorrhea if menarche has not occurred by age 16 as long as growth and sexual characteristic development are normal.
- Hypothalamic-pituitary defects cause decreased or non-pulsatile release of GnRH / LH / FSH and result in anovulation.
- Gonadal dysgenesis or other ovarian defects can result in anovulation.
- Gonadal dysgenesis results in fibrous "streaks" instead of functional ovaries.
- Turner syndrome is an example of a gonadal dysgenesis.
- Reproductive tract abnormalities account for 1/5th of primary amenorrhea cases.
[edit] Turner syndrome
- Turner syndrome is a missing or structurally abnormal X in an otherwise normal XX female.
- Turner syndrome patients have short stature and ovarian failure.
- Though the ovaries of patients with Turner syndrome initially develop normally, they undergo accelerated atresia during gestation.
- The fact that the ovaries develop normally but then degrade indicates that two healthy X chromosomes are required for maintenance of the ovaries.
- Turner syndrome often presents with primary amenorrhea or delayed puberty.
- The gonadotropins are elevated in Turner syndrome patients because there is no negative feedback via ovary-generated sex hormones.
[edit] Secondary amenorrhea
- Secondary amenorrhea means menstruation has ceased for over 6 months
- Possible causes of secondary amenorrhea include pregnancy, poly-cystic ovarian syndrome, and endocrine disorders.
- Endocrine disorders that cause secondary amenorrhea include:
- Hypothyroidism, hyperthyroidism
- Recall that TSH can cross-react with FSH receptor and thus cause precocious puberty.
- Hyperprolactinemia
- Non-classic Congenital adrenal hyperplasia
- Elevates androgens to the point of inhibiting LH / FSH production.
- Hypothyroidism, hyperthyroidism
- Polycystic ovary syndrome
- PCOS is the most common reproductive abnormality in women worldwide.
- PCOS results in chronic anovulism and hyperandrogenism.
- PCOS S&S include obesity and hirsutism.
- Note that hirsut means "hairy" in Greek or Latin.
- PCOS is often preceded by low birth weight and premature pubarche.
- Recall that pubarche is the development of pubic hair.
- PCOS generally occurs with many other metabolic problems, too; not the least of which is insulin resistance.
- STromal hyperthecosis is luteinizing of the theca cells and is correlated with circulating insulin levels.
[edit] Conclusions
- The HPO axis is complex and cyclic.
- Oocyte meiosis begins in prenatal life and arrests at prophase of meiosis 1 and again at metaphase of meiosis 2.
- Ovarian steroidogenesis requires proper functioning of theca cells and granulosa cells.
- The menstrual cycle is controlled by hormone changes and results in changes to the ovary and to the genital tract.
- Abnormalities of female reproduction can result in primary and secondary amenorrhea.
- stopped here on 04/07/11.
- started here on 04/08/11.
[edit] Pregnancy, parturition, and lactation
[edit] Learning objectives
- Understand the processes of fertilization and implantation
- Understand the structure and function of the placenta
- Be familiar with the endocrine changes that occur during pregnancy
- Understand key concepts of parturition
- Understand key concepts of lactation
[edit] Pregnancy
[edit] Female anatomy review
- The female reproductive tract is composed of gonads that generate gametes and a series of ducts that maintain the gametes and the fetus.
- The gonads are the ovaries, found bilaterally and suspended from the posteriolateral wall of the abdomen by the ovarian ligaments.
- The beginning of the duct system is the fallopian tube (oviduct) which catches the evicted ocum via fimbria and passes it to the uterus via the ampulla and isthmus divisions.
- The uterus has a fundus, corpus, and cervix; the inside surface of the corpus is the endometrium which is the location of implantation and the connection point between mother and child during pregnancy.
- The cervix connects to the vagina.
[edit] Fertilization
- Fertilization is defined as the successful union of sperm and an egg.
- For fertilization to occur, both the egg and the sperm must be rapidly transported to their union because they each have a lifespan of only 2 days.
- Sperm transport through the female reproductive tract is best facilitated during the follicular phase; more to the point, sperm travels best through the female reproductive tract during the proliferative stage of the uterine cycle.
- Recall that the proliferative stage of the uterine cycle is characterized by endometrial hypertrophy and formation of spiral arteries.
- For proper fertilization, fresh sperm must be present as the ovum enters the oviduct (Fallopian tube).
[edit] Sperm transport
- About 250 million sperm are released upon ejaculation, however, only 50 million will make it to the oviduct.
- Loss of sperm is due to: acidic pH of the vagina, phagocytosis of sperm in the uterus, and the anatomical barrier presented by the uterotubal junction (that is, where the isthmus of the oviduct dumps into the uterus is a small opening that the sperm must find and proceed through).
- Sperm arrive at the oviduct in less than 5 minutes!
- That's about 0.5 sperm body lengths / second.
- Sperm are able to move through the female ducts because of their own motility and because of assisting vaginal, cervical, and uterine contractions.
- Semen initially coagulates (bad for transport) in the ducts and then liquefies (good for transport).
- During the follicular phase, mucin molecules of the cervical canal are oriented in parallel to facilitate transport of the sperm.
[edit] Capacitation
- Recall the basic anatomy of the sperm: three parts (head, middle, tail), an acrosome (sac of enzymes in the head), a nucleus, mt, and a tail (axoneme).
- Capacitation is a series of irreversible changes occurring in the sperm as a result of being delivered to the female reproductive ducts.
- Capacitation results in increased motility and happens after an hour of being delivered to the ducts.
- Changes during capacitation include: loss of surface proteins and lipids and merging of acrosome with cell membrane.
[edit] Ovum transport
- Recall that during ovulation, the oocyte of the dominant follicle is ejected from the ovary.
- Recall the basic anatomy of the follicle: many layers, theca x 2, granulosa, antrum, fluid, corposa oophorus, corona radiata, zona pellucida.
- The ejection of the egg leaves leaves the theca and granulosa layers to become the corpus luteum
- This makes sense when one recalls that the corpus luteum will go on to be the primary source of hormones and the granulosa cells and theca cells generate estrogen / progesterone and androgens, respectively.
- The ejected egg will therefore be composed of the cumulus oophorus, the zona pellucida, and the cell body of the oocyte.
- This oocyte is collected into the fallopian tube (oviduct) by way of ciliary action of the fibria.
- Some additional oocyte anatomy:
- The oocyte has three pre-cellular layers as it encouters sperm: the cumulus oophorus and corona radiata surrounding the zona pellucida.
- Deep to the zona pellucida is the perivitelline space that separates the zona pellucida and the oolemma (the cell membrane of the oocyte).
- The oocyte also has a series of microvilli just underneath the oolemma, which are poised to be an important part of the egg's interaction with sperm.
- Finally, recall that the first polar body will exist with the oocyte within the protective encasing of the cumulus oophorus, corona radiata, and zona pellucida.
[edit] Events of fertilization
- Fertilization takes place in the ampulla of the oviduct.
- Recall that 50 million sperm have made it to the oviduct and 1 egg has been moved into the oviduct via ciliary action of the fimbria.
- Recall that the sperm has undergone capacitation to make it a better swimmer and to merge its acrosome with the cell membrane at the tip of the head section.
- Recall that the egg that makes it to the oviduct is covered by the cumulus oophorus, the corona radiata, the zona pellucida, the perivitelline space, and the oolemma.
- The process of fertilization can be view as the solution to two major tasks: 1) getting through the layers surrounding the oocyte to the cell body and 2) keeping multiple sperm from fertilizing the oocyte.
- Getting through the protective layers of the oocyte:
- When the sperm and egg unite, the sperm breaks through the cumulus oophorus and the corona radiata to adheres to the zona pellucida of the egg.
- Adherence of the sperm to the zona pellucida is mediated by the ZP3 receptor.
- Adherence to the zona pellucida triggers release of the enzymes of the acrosome.
- Enzymes of the acrosome digest the cumulus oophorus.
I don't understand how binding the zona pellucida can cause release of enzymes that digest the cumulus oophorus when the oophorus is superficial to the zona pellucida.
- The sperm penetrate the zona pellucida in about 15-30 minutes.
- Once through the zona pellucida, sperm cross the perivitelline space (space between the zona pellucida and the cell membrane of the oocyte).
- Upon adhering to the oolemma (cell membrane of the oocyte), microvilli of the oocyte extend to grasp the sperm and the spermatic head and tail are engulfed.
Really? I think it has been shown that the tail is very specifically NOT engulfed.
- Keeping multiple sperm from fertilizing the oocyte:
- Once the oocyte has grasped the first sperm to penetrate the zona pellucida, the egg undergoes changes to the oolemma that prevent other sperm from entering the cell.
- Penetration of the first sperm through the oolemma causes Ca to enter the cell and two processes to begin: cortical granule release and the completion of meiosis 2.
- Rising Ca levels cause cortical granule fusion with the oolemma.
- As the cortical granules fuse the zona pellucida undergoes changes such that we call it the zona reaction, which is impermeable to sperm.
- As meiosis 2 completes, a single oocyte nucleus (1N) remains.
- Finally, a zygote is generated by the fusion of the male and female pronuclei.
What's the 2-3 degree about?
[edit] Twinning
- Twinning occurs in 3% of live births.
- There are two types of twinning: monozygotic and dizygotic.
- Monozygotic twinning occurs when one zygote splits into two separate, functional embryos and generates identical twins.
[edit] Fertilization to implantation
- The first mitotic division of the embryo occurs within 24-36 hours of fertilization.
- This initial cell division occurs in the oviduct, near the site of fertilization.
- Note that the embryo continues to be surrounded by the zona pellucida for the first several division generations for several reasons:
- transport, protection, and inhibition of immune reactions.
- As the fertilized egg travels along the oviduct it will undergo mitosis and form into a solid ball of cells called a morulla.
- The morulla enters the uterus approximately 4 days after fertilization.
Is this after ovulation or after fertilization?
- It is possible that an egg can become fertilized outside of the female reproductive tract resulting in an ectopic pregnancy.
- Ectopic pregnancies can be life-threatening if not treated.
[edit] Implantation
- The process of implantation is about physically attaching to the endometrium and establishing communication between the embryo and the mother's vascular system.
- A blastocyst implants in the endometrial wall of the uterus at approximately 7 days after fertilization and is fully embedded by 8-12 days post-ovulation.
- A blastocyst is a collection of 20-30 cells that have two distinct regions (the trophoblast and the embryoblast) with a fluid filled center.
- The trophoblast will go on to become all the extraembryonic tissue like the plaenta and umbilical cord.
- The embryoblast (inner cell mass) will go on to become the fetal tissue.
- The cells of the trophoblast commence the process of implantation by using proteases to invade the endometrium.
Is there a consistent location within the uterus for implantation? Does the location of the placental attachement change along the way? Does it effect parturition?
- The trophoblasts embedded in the endometrium differentiate into two cell populations to facilitate development of communication between the embryo and the mother's vascular system.
- Cytotrophoblasts: become villi and eventually chorionic villi.
- Syncytiotrophoblasts serve to increase the surface area for embryo-mother interaction and also secrete progesterone and hCG.
- Upon trophoblast invasion, the endometrium becomes undergoes changes to become a decidua in a process called the decidual reaction.
- The maintenance of the decidua requires progesterone from the luteal cells of the corpus luteum and the synciotrophoblasts.
- The decidua is characterized by large, polyhedral, multinucleated cells, dilated blood vessles, and lacunae.
- Eventually, the embryo will be composed of the three embryological tissue types: ectoderm, endoderm, and mesoderm.
[edit] Methods of contraception
- Pregnancy can be prevented at several points along the pathway of baby-generation: ovulation, fertilization, implantation.
- Preventing fertilization:
- Coitus interruptus is the removal of the penis from the female reproductive tract before ejaculation; coitus interruptus prevents fertilization by minimizing or preventing delivery of sperm to the female genital tract.
- The rhythm method of preventing fertilization relies on the timing of intercourse along the ovarian / uterine cycle. Since egg and sperm can only successfully fertilize during a several-day time frame (just after ovulation, before either cell degenerates), it is possible to time intercourse so that viable gametes do not come in contact.
- Barrier protection (like condoms and IUDs) can inhibit the transfer of sperm to the female genital tract and thus inhibit fertilization.
- Preventing ovulation:
- Oral contraceptives provide estrogen to inhibit LH and FSH through negative feedback and thus inhibit development of the follicle.
Do oral contraceptives really "adversely affect the environment of the tract"?
- Morning after pill provides high levels of progestin or progestin and estrogen to ....
How does high levels of progestin / estrogen inhibit ovulation?
- Preventing implantation:
- RU-486 provides long-acting progesterone, high doses of estrogen, or progesterone-receptor antagonists....
How does RU-486 work?
- High levels of prolactin (which can be maintained through regular breast-feeding) can suppress pulsatile release of GnRH and therefore prevent ovulation and pregnancy.
- Recall that GnRH from the hypothalamus stimulates LH / FSH release and that LH / FSH serve to mature the follicle and thus regulate ovulation.
- Note that one cannot assume that regular breast-feeding will prevent pregnancy.
[edit] Function of the placenta
- The whole point of the placenta is to exchange nutrients and waste, including:
- Oxygen / CO2 (via gradient, enhanced by HbF)
- Glucose, aa, electrolytes, hormones, etc.
- These are exchanged via diffusion, facilitated diffusion (non-energy dependent channels), and pinocytosis.
- Because the placenta effectively provides a blood-placental barrier, it can provide differential exchange.
What is differential exchange?
- It should be noted that not all drugs cross the placental barrier but most viruses do cross the barrier.
[edit] Structure of the placenta
- After implantation, the trophoblast offspring (cytotrophoblasts and synciotrophoblasts) interdigitate with endometrial cells to form chorionic villi and lobes called cotyledons.
- Recall that the placenta is designed to facilitate nutrient exchange between the mother and child.
- The placenta is said to be hemochoroidal because blood of the baby is brought to and from the villi (where it runs very near to the mother's blood) via two umbilical arteries and one umbilical vein.
- The fetal endothelium and connective tissue are bathed in mother's blood at the villi.
- The placenta has two layers: the aminon (inner-most) and the chorion (outer-most).
[edit] Endocrinology of pregnancy
- Make no mistake, the placenta is a temporary endocrine organ; the placenta produces several important hormones for the maintenance of pregnancy.
- The placenta produces progesterone, estrogen:
- Recall that estrogen and progesterone from the follicle and corpus luteum (follicular and luteal phases of the ovarian cycle) conditioned the female genital tract (uterus, cervix, vagina) for fertilization and implantation (the proliferative and secretory stages of the uterine cycle).
- So, it makes sense that the placenta would make progesterone and estrogen in order to maintain the microenvironment of the genital tract.
- The placenta produced human chorionic gonadotropin:
- hCG is produced by trophoblast cells of the placenta.
- hCG is an alpha-beta protein (alpha constant, beta unique) just like LH and FSH.
- hCG binds the LH receptors.
- Recall that the corpus luteum has granulosa cells and theca cells; recall, too, that theca cells have LH receptor throughout development of the follicle and granulosa cells begin to express LH receptor as the follicle matures to ovulation.
- Therefore, it makes sense that hCG is called a gonadotropin because hCG extends the life of the corpus luteum and elevates production of progesterone (by the reciprocal action of granulosa and theca cells).
- hCG peaks at 10-15 weeks.
- We suspect that hCG has a critical role throughout pregnancy.
- The placenta produces placental lactogen:
- hPL is produced by syncitiotrophoblasts of the placenta.
- hPL promotes milk-production (lactogenic) and somatic growth (growth-hormone-like).
- hPL ensures adequate fuel supplies for the
parasitefetus by reducing mother's glucose use and mobilizing mother's adipose reserves.
- The placenta produces other peptides:
What do I need to know about "other peptides"?
[edit] Steroid hormones of pregnancy
- The two major steroid hormones of pregnancy are progesterone and estrogen.
- Recall that progesterone and estrogen serve to condition the genital tract microenvironment for pregnancy.
- Progesterone:
- Produced for the first 8 weeks primarily by the corpus luteum.
- Recall that the trophoblasts of the placenta produce hCG which is a gonadotropin that maintains the life of the corpus luteum beyond the 2 weeks it would normally produce progesterone / estrogen while degrading during the luteal phase of the ovarian cycle.
- After 8 weeks, the trophoblasts of the placenta are the primary producers of progesterone.
- Trophoblasts have LDL receptors that collect LDL cholesterol from the mother's blood supply and convert it into progesterone.
- Progesterone levels rise throughout pregnancy.
- Progesterone conditions the genital tract in three ways: inhibits utrine contraction, inhibits prostaglanding formation, and inhibits immune response at the uterus.
- Specifically, progesterone binds to uterine smooth muscle cells to inhibit contraction.
- Specifically, progesterone inhibits T cell response at the uterus to provide temporary immune privilege to the uterus.
- Produced for the first 8 weeks primarily by the corpus luteum.
Why does it make sense that progesterone would inhibit prostaglandins? Would you want increased blood flow at the uterus?
- Estrogen / estradiol:
- Recall that estrogen is produced by the granulosa cells of the corpus luteum throughout the luteal phase (~2 weeks w/o pregnancy, extended to 8 weeks with hCG from placenta).
- Production of estrogens require a functional fetoplacental unit (that is, a functional connection between the fetus and the mother).
- Estrogen levels rise throughout pregnancy just like progesterone.
- Estrogen serves to condition the mother's genital tract: increases uterine size and increases uterine blood flow.
- Estrogen also affects fetal development; estrogen augments organ development and affects breast and adipose development.
- Lastly, estrogen is involved in implantation.
To what extent?
[edit] Maternal changes of pregnancy
- The physical presence of the growing fetus, along with estrogen, progesterone, and human placental lactogen cause changes to the mother's physiology.
- Recall that progesterone inhibits contractions, inhibits prostaglandins, and inhibits immune response at the uterus.
- Progesterone's inhibition of uterine contractions affects other smooth muscle, too, causing gastroesophageal reflux and constipation.
- Recall that the lower esophageal sphincter is smooth muslce (lower 1/3 is all smooth muscle) and the colon requires periodic smooth muscle peristalsis to move feces along so they do not dry out.
- Progesterone also causes increased minute ventilation.
- Progesterone's inhibition of uterine contractions affects other smooth muscle, too, causing gastroesophageal reflux and constipation.
- Recall that estrogen causes uterine growth and increased uterine blood flow.
- Estrogens cause increased blood flow systemically which can lead to sinus congestion, bleeding gums, and sensations of warmth.
- Estrogens cause increased perfomance of the cardiovascular system in general: increased blood volume, increased cardiac output, and increased heart rate.
- Recall that hPL (human placental lactogen from the synciotrophoblasts) causes milk production, reduced glucose use and increased adipose energy store mobilization in the mother.
- The mother will gain weight to ensure enough energy for fetal peak growth and in the case of starvation.
[edit] Endometriosis
- Recall that endometrial cells line the inside of the uterus and respond to estrogen and progesterone by proliferation.
- When endometrial cells exist outside of the uterus (like on the ovaries), they can proliferate and cause pathology.
- S&S include pelvic pain that worsens with the menstruation cycle.
- It makes sense that pain would increase during during the follicular uterine cycle and the luteal phase of the ovarian cycle because estrogen levels will be high.
- Endometriosis is in 5-10% of women--usually during the reproductive years.
- Endometriosis is a common cause of infertility.
[edit] Parturition
- Parturition is a poorly understood concept in general.
- We do know that there are both fetal and maternal factors that play a role.
- We know that there are both hormonal and mechanical factors.
- We suspect that paracrine factors may be the most important kind of hormone signaling involved in parturition.
- There are 5 major hormones that affect parturition: estrogen, progesterone, oxytocin, relaxin, placental-corticotropin releasing hormone (pCRH).
- We know that estrogen is generally pro-parturition and progesterone is generally anti-parturition.
- Estrogen's role in parturition:
- Recall estrogen is generally pro-parturition.
- Progesteron's role in parturition:
- Recall that progesterone is generally anti-parturition.
- In many species progesterone declines sharply just before parturition, however we don't know if progesterone decreases significantly in humans.
Is this the case?
- Recall that progesterone inhibits prostaglandins during pregnancy.
- During parturition, there is a sharp increase in prostaglandins in the amniotic fluid.
- So, we suspect that decreased progesterone during parturition allows increased prostaglandins.
- Increased amniotic prostaglandins stimulate contractions and cervical ripening.
- Recall that progesterone inhibits prostaglandins during pregnancy.
- Oxytocin's role in parturition:
- Oxytocin augments labor but doesn't increase uterine responsiveness or density (that is, it doesn't cause uterine contractions to be more forceful or more common).
- Recall that myometrial cells (uterine smooth muscle cells) can coordinate via gap junctions.
- Cervical and vaginal dilation causes explosive, pulsatile release of oxytocin during parturition.
- We believe that this release of oxytocin developed to help mother's deliver the subsequent litter-mates (and in humans the placenta).
- Note that women with a posterior pituitary defect proceed through parturition normally.
- Recall that the posterior pituitary releases oxytocin generated by the neurons of the nuerohyophysis.
- Oxytocin augments labor but doesn't increase uterine responsiveness or density (that is, it doesn't cause uterine contractions to be more forceful or more common).
- The role of relaxin is in parturition is unclear.
- pCRH's (placental corticotropin releasing hormone) role in parturition:
- pCRH (placental corticotropin releasing homrone) seems to affect the estrogen-progesterone ratio in sheep and humans.
- In general, pCRH increases exponentially near parturition.
- pCRH shifts production from progesterone to estrogen.
- This makes sense; recall that estrogen is pro-parturition and progesterone is anti-parturition.
- Recall that progesterone inhibits prostaglandins; so it makes sense that elevated pCRH is associated with elevated prostaglandins because pCRH causes a relative decrease in progesterone to estrogen.
- Additional evidence of the importance of pCRH:
- Sheep without anterior pituitary function or adrenal function (that is, lacking ACTH and cortisol) have longer gestation periods (that is, delayed parturition).
- Women who deliver early show elevated levels of pCRH within 24-48 before parturition.
[edit] Lactation
- Lactation is the production of milk by a woman.
- Lactation is primary controlled by the maternal pituitary gland.
- Recall that lactotropes (mammotropes) of the anterior pituitary produce prolactin.
- Recall that neurons of the neurohypophysis (posterior pituitary) produce and release oxytocin.
- Lactation requires suckling upon the nipple to be maintained; that is, neural sensation at the nipple is required for milk production by the mammary glands.
- Maternal milk is the "perfect nutrition source" for newborns because it is easy to digest, sterile, and the perfect temperature.
- Breast-milk infers several health advantages on the child: decreased risk of obesity, decreased ear infections, decreased food allergies, decreased GI illnesses
[edit] Development of the mammary glands
- Mammary gland precursor cells develop along the bilateral mammary ridges in both boys and girls.
- Accessory nipples can result from poor degenerative signaling that usually limits nipple development to just two, symmetrical nipples.
- There is an initial development and maturation of the mammary glands throughout fetal and post-natal development.
- This initial development is identical in boys and girls and allows the glands to be responsive to hormonal signals.
- Note that this development stage sets the mammary glands up to either be signaled to develop further by the relatively high estrogen levels pregnancy or to remain under-developed in boys.
- Secondary development is characterized by the change of connective tissue to milk ducts and occurs in response to hormone elevation during pregnancy.
What is witch's milk in newborns?
[edit] Anatomy of the breast
- Breast tissue contains epithelial milk producing cells arranged in alveola, myoepithelial cells surrounding the alveoli, and epithelial lined tracts.
- Cuboidal epithelial cells that make up the alveoli are signaled by prolactin to produce milk and secrete it into the epithelial secretory alveoli (the beginning of the ductule system).
- Myoepithelial cells around the alveoli are signaled by oxytocin to contract, thus squeezing milk along the tracts.
- The epithelial ductule system of mammary glands begins as the tract within alveoli called secretory alveoli, progresses to intralobular ducts, and then to collecting ducts that converge on the nipple.
[edit] Characteristics of human milk
- The characterisitics of human milk change over a short time frame post-parturition.
- The initial milk produced is called colostrum and is high in lymphocytes and antibodies which is good for helping the baby deal with its new, infectious environment.
- The milk matures from colostrum into hindmilk within three days.
- Hindmilk contains higher fat content which promotes satiety in the newborn.
- Note that pregnancy and lactogenesis are well coordinated because lactogenesis occurring with pre-term delivery generates milk with a higher protein and fat concentration which makes sense because the newborn has some extra growing to do.
[edit] Lactogenesis
- Milk production begins in the 5th (~20 weeks) month of gestation.
- Note that only colostrum is produced before and immediately following parturition.
- Production of hindmilk is inhibited by high progesterone and estrogen levels of pregnancy.
- Recall that estrogen and progesterone are produced by the placenta so it is not until the placental source is arrested (via parturition) that hindmilk can be produced.
- Progesterone and estrogen are prolactin antagonists.
[edit] Role of prolactin
- Recall that prolactin is produced by lactotrophs of the anterior pituitary.
- Estrogen increases prolactin production during pregnancy. (Recall that estrogen levels rise throughout pregnancy and that estrogen is a prolactin antagonist).
- Note that estrogen increases prolactin levels but decreases prolactin's ability to have an effect at the mammary gland tissue.
- Recall that dopamine is constantly expressed in non-pregnant females to inhibit prolactin production at the anterior pituitary.
- Estrogen increases prolactin production during pregnancy. (Recall that estrogen levels rise throughout pregnancy and that estrogen is a prolactin antagonist).
- Prolactin has several effects at the mammary tissue: increases milk production, stimulates lactose production, and stimulates expression of milk-protein genes.
- Prolactin production increases at each nursing (that is, it is produced as a result of suckling stimulation).
[edit] Role of suckling
- An infant suckles on the nipple in a back-to-front action.
- Stimulation at the nipple by an infant has a short term and a long term effect via neuronal-hormonal reflex arcs: milk secretion and milk production.
- The afferent (at the CNS) signal for these reflex arcs is physical stimulation of the neurons that innervate the nipple.
- The short term, milk-secretion arc occurs through neuronal secretion of oxytocin (the hormonal efferent--exit the CNS signal) from the neurohypophysis (posterior pituitary) which stimulates the myoepithelial cells surrounding the mammary gland alveoli to contract, secreting milk for the infant.
- The long term, milk-production arc occurs through increased prolactin production (the hormonal efferent--exit the CNS signal) which causes increased milk production by signalling the epithelial cells of the mammary gland alveoli.
- Milk-let down is the release of milk from the lactiferous ducts to the lactiferous sinus.
- The let down effect may be secondary to CNS effects (meaning stimulation at the nipple gets to the posterior pituitary via the CNS and causes oxytocin release).
[edit] Role of oxytocin
- Oxytocin has social-behavioral effects in addition to the direct physical effects previously described (think myoepithelial signaling, milk-secretion, dilation of cervix and vagina).
- Indeed, oxytocin has been considered a love potion.
- Oxytocin acts as a nuerotransmitter in the brain and is associated with maternal nurturing behavior, trust, decreased fear, increased empathy.
- Oxytocin is also associated with female orgasm and a habit of long-term mating.
[edit] Sustaining lactation
- Lactation can be maintained for years after parturition given regular stimulation of the nipple.
- The supply of milk produced by the mother is determined by the need of the baby.
- Maximum volume production falls within 800-1200 ml / day.
- High levels of prolactin (which can be maintained through regular breast-feeding) can suppress pulsatile release of GnRH and therefore prevent ovulation and pregnancy.
- Recall that GnRH from the hypothalamus stimulates LH / FSH release and that LH / FSH serve to mature the follicle and thus regulate ovulation.
- Note that one cannot assume that regular breast-feeding will prevent pregnancy.
[edit] Summary
- Steroid hormones of pregnancy produced with maternal, fetal and placental contributions
Where did we see fetal contribution?
- Human parturition poorly understood
- Lactation involves hormonal regulation as well as neural response to infant suckling
- stopped here on 04/07/11.
- started here on 04/08/11.
[edit] Regulation of body temperature
[edit] Objectives
- The student will be able to describe the balance between metabolic heat production and heat loss.
- The student will be able to describe the 5 mechanisms whereby body heat is lost.
- The student will be able to describe how heat is transferred to the environment.
- The student will understand the factors that affect body temperature and how the body regulates temperature in narrow limits, despite these factors.
- The student will be able to describe how acclimatization to environmental temperature is achieved.
- The student will be able to describe metabolic rate and list the factors affecting it.
[edit] The range of body temperature
- At rest, the normal rectal temperature range is 36-38 C.
- During exercise the normal rectal temperature is 38-41 C.
- Heatstroke and brain lesions cause rectal temperatures 41-44 C.
- Temperature regulation is said to be impaired when temperatures are below 36 or above 41.
- Different areas of the body are maintained at different temperatures.
- Warmest -> coolest: rectum, head, trunk, skin, hands, feet.
- Body temperature fluctuates, following a circadian (per day) rhythm.
- Note that hyper or hypo thyroidism shifts the curve up or down, respectively, but does not change the rhythm of fluctuation.
[edit] Heat production and loss
- There are a handful of variables that effect the production and loss of heat.
- Production: basal metabolism, muscular activity, thyroxine effects, epinephrine effects, temperature effects.
- Loss: radiation, evaporation, conduction-convection
- When production is elevated over loss, one becomes hyperthermic; conversely, when loss is elevated over production, one becomes hypothermic.
[edit] Metabolism as a heat producing process
- Energy exists in food as chemical energy.
- Our convert whole food into a pool of metabolic chemical energy, then as chemical energy within cells, and then as work.
- At each step, heat is generated.
- Work comes in several forms: osmotic, mechanical, electrical, chemical.
- The human body uses about 20% of the chemical energy in food for work.
- Note that a Calorie (big C) is the same as a kcal; that is, one Calorie is 1000 calories.
- We define metabolic rate as the amount of food energy converted per unit time.
- Metabolic rate could also be defined as the total daily energy expenditure.
- There are many factors that affect the metabolic rate (total daily energy expenditure): exercise, thermogenic effect of food, hormones, body size, age, gender, disease, and ambient temperature.
- Exercise: the more exercise, the more calories needed each day; can range up to 4800 cal / day for hard physical labor.
**Thermogenic effect of food: we generate heat by processing food so
- Hormones: catecholamines and thyroid hormones can be used to regulate metabolism
- Catecholamines increases glycogen breakdown
- Thyroid hormones increase heat by increasing metabolism of glucose and fat.
- Body size: the metabolic rate is proportional to the surface area of the animal due to loss of heat at the surface area.
- Large animals generally have slower metabolisms because they lower surface-area-to-volume ratios and proportionally less heat loss at the surface.
- Age / gender: younger and males generally have higher metabolisms
- Younger pts are synthesizing more products so must have higher metabolisms.
- Hormones: catecholamines and thyroid hormones can be used to regulate metabolism
***We don't know what the subcutaneous fat in females is all about.
- Disease: metabolic rate is often increased to inhibit infections
- A new metabolic rate is set and thus a fever occurs.
- Ambient temperature: cold environments can lead to elevated metabolic rate
- A new set point is set by elevating the thyroid hormones.
- Disease: metabolic rate is often increased to inhibit infections
[edit] Body temperature measurements
- The body is said to have a homeothermic core that is kept a constant temperture.
- The body also has a poikolothermic shell; poikilothermic means "changes with the environment".
- The poikilothermic shell does vary with the environment yet is maintained by the core source of heat.
- We measure body temperature in three ways: the core temperature, the mean skin temperature, and the mean body temperature.
- The mean body temperature is a composite of the core temperature and the mean skin temperature.
- The core body temperature:
- Core body temperature is normally 37.5 C.
- Core body temperature can be measured rectally (RT), orally, or axillary-ly.
- The core temperature has a normal range and is measurement dependent.
What does "measurement dependent" mean?
- The mean skin temperature (MSC):
- The mean skin temperature is a composite of four separate measurements: chest, arm, thigh, and calf.
- The MST has a normal variance and is dependent on the muscle mass beneath the location of measurement.
- The equation for calculating the MSC combines the four measurements with coefficients.
- MST = 0.3 (chest + arm) + 0.2 (thigh + calf)
- MST = 0.3 (32 + 36) + 0.2 (34 + 31)
- MST = 20 + 13
- MST = 33C
- The mean body temperature (MBT):
- The mean body temperature is a compsite of the core body temperature and the mean skin temperature (MSC).
- MBT = 0.3 (MST) + 0.7 (RT)
- MBT = 0.3 (33) + 0.7 (37.5)
- MBT = 35.9
[edit] Heat transfer
- Heat transfers from the core to the poikilothermic shell via convection much more effectively than conduction.
- The circulatory system moves heat from the core to the shell via convection.
- Note that conduction is not efficient through tissue.
- The only exception is the vasculature which is actually a very good conductor.
[edit] Evaporative heat loss
- We lose heat through evaporation: both sensible (sweating) and insensible.
- Sensible evaporative heat loss (sweating) can lose up to 1500 mL / hour in extreme conditions.
- Insensible evaporative heat loss generally costs about 600 mL / day.
- Note that diffusion provides the constant source of water at the skin surface to be evaporated off.
- It takes about 0.58 Kcal of energy to cause each gram of H20 to evaporate.
[edit] Heat dissipation
- Recall that the metabolic rate (M) is the total production of energy from intake (diet).
- We can define the "rate of heat storage (S)" as the metabolic rate (M) minus all the sources of heat loss.
- Source of heat loss include radiative heat loss (R), convective heat loss (C), and evaporative heat loss (R).
- So we say: S = M - R - C - E
What about conduction? and aren't evaporative and convective some what dependent?
[edit] Active regulation of heat transfer
- Recall that the skin has cold and warm receptor fibers.
- Feedback by these receptors can help the brain regulate heat transfer.
- Warm fibers fire more and more rapidly beginning at 30 C (and ending at 46ish).
- Cold fibers fire more and more rapidly beginning at 43 C (and decreasing again at 27ish).
[edit] Active regulation of body temperature
- Peripheral and central thermoreceptors provide information to the CNS which integrates the information at the hypothalamus.
- The feedback provided by the thermoreceptors is negative feedback.
- Peripheral and central thermoreceptors:
- Peripheral cold and warm thermoreceptors increase their firing rate upon appropriate sensation.
- We have 10 times as many cold receptors as we do warmth receptors.
- Central core cold and warm receptors are found in the viscera and send input to the brain.
- The central and peripheral thermoreceptors report back to a single controller.
- Peripheral cold and warm thermoreceptors increase their firing rate upon appropriate sensation.
- CNS integration of feedback:
- The hypothalamus is the main controller and maintains a "metabolic set point" and "skin set point".
- The hypothalamus sends efferent signals to the skin, sweat glands, and muscle upon integration of afferent signals from central and peripheral thermoreceptors.
- The CNS responds to an increase in "warm" feedback: vasodilation and sweating.
- This is called the antirise response.
- The CNS responds to an increase in "cold" feedback: vasoconstriction and shivering.
- This is called the antidrop response.
- The hypothalamus is the main controller and maintains a "metabolic set point" and "skin set point".
[edit] Pathologic temperature regulation
- Fever:
- Fever occurs as a mechanism to fight infections.
- Pyrogens released by the immune system can cause an elevation in the hypothalamic set point.
- Pyrogens can include cytokines or bacterial products.
- Once the pyrogen is gone, the set point returns to normal.
See what he says on slide 15.
- Hyperthermia:
- Hyperthermia is an upregulated temperature increase; that is, an abnormal storage of heat in the body core.
- Hyperthermia is most often caused by prolonged exposure to high temperature combined with high humidity.
- Note that humidity increases the effect of heat on the body because the body is less able to lose heat by evaporation (because the vapor pressure is high so water doesn't want to leave the body surface).
- Heatstroke is defined as the "breakdown of heat loss capabilities".
- Heat exhaustion is defined as "hypotension due to excessive loss of fluid via sweating"; this is an example of overactive heat loss.
- Hypothermia:
- Hypothermia is defined as "unregulated temperature decrease".
- In hypothermia, heat production and conservation capabilities are exceeded by environmental cold.
[edit] Temperature elevation in fever versus exercise
- Note that though both fever and exercise see an elevation of body temperature, in exercise the set point has not changed so there is a constant signaling saying "hey! it's too hot in here".
What else is the point on slide 16?
[edit] Environmental temperature acclimatization
- The body is able to acclimatize to heat by assuming a lower basal rectal temperature, a lower heart rate, and a larger sweat response.
- The body is able to acclimatize to cold by increasing the basal metabolic rate, increasing tissue insulation, and producing a larger cold-induced vasodilation response.
- Recall that BMR (basal metabolic rate) is higher for both genders early in life when the body is growing.
- Recall that BMR is higher for men.
[edit] Measuring BMR
- BMR can be measured by direct or indirect calorimetry.
- Direct calorimetry:
- Direct calorimetry should take place under standard conditions: pt has fasted for 12 hours, early morning test (to measure lowest daily metabolic rate), room should be 25C, pt should be given 30-60 minutes of rest before the test.
- The pt is then put in a small, well-insulated room.
- Water is moved through a radiator in the room and measured before and after exposure to the room.
- Knowing that 1 kcal heats 1 gram of H20 1 C, one can calculate how many kcal of heat the pt is generating.
- Direct calorimetry results can be compared between separate individuals.
- Indirect calorimetry
- Indirect calorimetry uses oxygen consumption to derive the metabolic rate.
- Recall from biochemistry that carbs, fat, and protein all generate a certain amount of energy per oxygen molecule (because they all end up generating energy for the ETC which burns oxygen).
- Carbs generate 5.0 kcal / L of O2.
- Fats generate 4.7 kcal / L of O2.
- Protein generates 4.6 kcal / L of O2.
- On average, "food" generates 4.825 kcal / L of O2.
- Indirect calorimetry requires a pt to breath a closed volume of oxygen over several minutes.
- Measurements of the oxygen concentration are taken over time and thus rate of oxygen consumption can be generated.
- With a rate of oxygen consumption (volume) and the knowledge that the pt's input generates 4.825 kcal / L of O2 we can calculate the metabolic rate.
- MR = (rate of oxygen consumption * 4.825 kcal / L) / meter2
- MR = *L of O2 / Hr * 4.825 kcal / L) / m2
- MR = 40 kcal / m2 / hr
- stopped here on 04/08/11.
- started here on 04/11/11.
[edit] Exercise physiology
[edit] Objectives
- The student will be able to describe the 3 metabolic systems that supply energy during exercise and relate exercise conditions with nutrient fuel use.
- The student will understand how oxygen consumption varies with exercise intensity.
- The student will be able to describe the 2 stages of oxygen recovery.
- The student will be able to describe respiratory changes during exercise.
- The student will be able to describe chemical and neural mechanisms stimulating ventilation during exercise.
- The student will be able to describe the dynamic relationship between changes in stroke volume and heart rate during exercise.
- The student will be able to describe the redistribution of blood flow to muscles and other organs during exercise.
- The student will understand the unique regulation of temperature during exercise.
- The student will understand the effect of training on cardiovascular, respiratory and metabolic function.
[edit] Metabolic aspects of exercise
- Exercise requires lots of ATP for all the work the muscle is doing.
- ATP can be generated in three ways: the phosphagen system, the glycogen-lactic acid system, or the aerobic system.
- The phosphagen system uses creatine kinase to move the phosphate group off of creatine to ADP, generating ATP.
- The phosphagen system (and the resident ATP) covers the energy for the 0-60 seconds of vigorous exercise.
- The glycogen-lactic acid system runs glycogen through glycolysis to generate lactic acid.
- The glycogen-lactic acid system covers the energy for the 1-4 minutes of vigorous activity.
- The aerobic system uses the electron transport chain to generate ATP from glucose, fatty acids, and amino acids.
- Aerobic oxidation of muscle glycogen, plasma glucose, and liver glycogen cover the energy for minutes 4-200 (and then tapers off).
- Aerobic oxidation of plasma FFA (free fatty acids) and adipose tissue TAGs (triacylglycerides) cover the energy for minutes 45 and beyond.
- The phosphagen system uses creatine kinase to move the phosphate group off of creatine to ADP, generating ATP.
[edit] Energy conversion in skeletal muscle
- Recall that glycolysis takes glucose to two pyruvate molecules, generating 6 ATP and 2 NADH.
- Recall that bursts of heavy activity utilize the phosphagen system and the glycogen-lactic acid systems for production of ATP.
[edit] Energy suply to muscle during exercise
- During exercise, epinephrine is elevated which signals to the liver, skeletal muscle, and adipose tissue.
- Epinephrine at the liver:
- Epinephrine causes the liver to increase glycogenolysis and gluconeogenesis.
- Epi--like glucagon--binds to a receptor that elevates cAMP levels and thus triggers activation of appropriate enzymes.
- Note that gluconeogenesis can use lactic acid as a precursor to be converted into glucose.
- Epinephrine at the muscle:
- Epinephrine at the skeletal muscle signals for the use of glycolysis (the anaerobic burning of glucose).
- Epinephrine binds to a cAMP elevating receptor on skeletal muscle which leads to activation of appropriate enzymes for converting glucose into ATP and aerobic intermiediates (think NADH and pyruvate).
- Glucose converted to pyruvate too quickly to be used in the (limited capacity citric acid cycle--oxphos) can be converted to lactic acid and secreted into the blood to be used in gluconeogenesis at the liver.
- Recall that this loop (glucose -> pyruvate (to get the ATP and NADH) -> lactic acid -> liver -> glucose -> muscle -> pyruvate...) is called the cori cycle.
- Epinephrine at the adipose tissue:
- Epinephrine at the adipose tissue causes TAG breakdown into FFAs for secretion into the blood.
- Epinephrine binds to a receptor that activates the hormone-sensitive lipase.
[edit] Oxygen consumption during exercise
- The basal rate of oxygen consumption is about 0.25 L / minute.
- Light exercise can elevate oxygen consumption 3-fold to about 1 liter.
- Heavy exercise can elevate oxygen consumption 8-10 fold to nearly 3.5 liters.
[edit] VO2 max: Maxiumum O2 consumption
- The VO2 max is the point at which oxygen uptake and transport are at their maximum capacity.
- The VO2 max can be reached upon heavy exercise of large muscle groups for over three minutes.
- At VO2 max, the blood lactic acid levels have reached over 8 mM.
- Recall that pH has an effect on Hb's oxygen carrying ability so these high levels of lactic acid inhibit continued elevation of oxygen transport.
- O2 consumption at VO2 max is at its maximum even if exercise continues at a higher rate; that is, one can consume energy at a higher rate than the VO2 max allows for production of energy, but the difference in will come from anaerobic energy sources.
- Factors affecting VO2 max include age, gender, and training level.
- The younger the higher the VO2 max.
- Men have higher VO2 max points than women.
- Training can increase the VO2 max.
How?
- Physiological limitations to VO2 max:
- You have to get the oxygen to the muscle, so the cardiovascular system can limit the VO2 max by how well blood flows to the muscle.
- Efficiency in O2 use determines how much O2 is needed for a given output; the more efficient the mitochondrial enzymes of the muscle, the higher the VO2 max can reach.
- You have to get the oxygen into the blood, so pulmonary diffusion can limit the VO2 max; the faster / better the pulmonary diffusion, the higher the VO2 max can reach.
[edit] Oxygen debt
- Oxygen debt is the idea that when oxygen demand increases rapidly (as in the case of exercise), too little oxygen is consumed during the initial phase as the body compensates (i.e. increased respiration and circulation).
- The debt from this initial phase is "paid off" by a lingering oxygen demand beyond the period of exercise.
- The "pay off" has two components: the fast component and the slow component.
- The fast component restores oxygen stores.
- The slow component finishes metabolism of lactic acid into waste or glucose.
- The "pay off" has two components: the fast component and the slow component.
[edit] Exercise intensity, duration, and fuel availability dictate exercise nutrient use
- We previously saw that different energy sources were used as exercise moved from short to prolonged, and thus the duration can affect the energy use.
- Intensity affects energy source: light exercise energy needs are met by lipid metabolism while high-intensity exercise energy needs are met by metabolism of carbohydrates and protein.
- Lipids are burned for light exercise.
- Carbs and protein are burned for high intensity and long duration exercise.
- The diet, too, can affect which fuels are used during exercise.
- A high carbohydrate diet dictates a higher pecentage of the energy used during exercise be from carbs; similarly, a high fat diet dictates that more energy come from fat..
- Note that a high-fat diet has the earliest exhaustion (as in exhaustion of available energy sources) point, then a mixed diet, and then a high-carbohydrate diet.
What is that steady state point all about?
[edit] Diet can affect how glycogen recovers after exercise
- Diet can affect how glycogen stores are recovered ‘’’after’’’ exercise, too.
- Muscle glycogen replacement after depletion via exercise and best with a high carbohydrate post-exercise diet.
- Fat and protein diets are comparable to eating no food at all in terms of glycogen store restoration.
[edit] Respiration during exercise
- Exercise increases the oxygen demand of the body; the lungs meet the elevated demand by ‘’increasing the rate of respiration’’’, ‘’’increasing the volume of respiration’’’, and ‘’’increasing the diffusion capacity of the lung’’’.
- The respiratory rate can increase 4-fold to 50 breaths / min.
- The respiratory volume can increase 6-fold to 3000 mL / breath.
- The diffusion capacity can incrnease 4-fold to 80 mL / mmHg.
How does the diffusion capacity increase? Vasodilation, right? Anything else?
- Through increased rate and volume, total ventilation (TV) can be elevated nearly 25-fold!
- TV = mL / min
- TV = rate * volume
- TV = breaths / min * mL / breath
- TV (rest) ~= 12 breaths / min * 500 ml / breath = 6000 mL / min = 6 L / min
- TV (work) ~= 50 breaths / min * 3000 ml / breath = 150000 / min = 150 L / min
[edit] Control of ventilation during exercise
- Ventilation elevation (‘’’hyperpnea’’’) during exercise occurs in ‘’’three stages’’’: fast, slow, and steady state.
- Fast stage: ventilation is immediately and rapidly elevated.
- Slow stage: ventilation continues to be elevated but at a slower, attenuated rate.
- Within 2 minutes of commencing exercise, the slow stage is nearly half-way complete.
- Steady state stage: ventilation is maintained as needed.
- Within about 4 minutes of beginning constant exercise, the steady state has been met.
[edit] Respiratory stimuli
- Recall that there are chemoreceptors that measure blood pH, PCO2, and PO2 and provide stimulation to the CNS.
- However ‘’’these chemoreceptors do not account for the respiratory response to exercise’’’.
- It is posited that ‘’’feed foward regulators of ventilation may activate neural reflexes’’’ to cause elevated respiration in response to exercise.
- Beyond these chemoreceptors, there are several neural stimuli that may contribute to respiration elevation during exercise.
- motor cortex efferent (that is, the signals ‘’’from the locomotive forebrain’’’ that are leaving the brain telling the body to move) may contribute to respiration control
- proprioceptors (which are also called ‘’’ergoreceptors’’’) send afferent signals to the brain as perception of where in space the limbs reside and how much tension is on the muscles; these afferent signals may be a source of respiratory control
- lung stretch receptors and vascular stretch receptors (which would be activated during exercise) send afferent signals to the brain and may therefore affect exercise respiration
**elevated sensitivity to respiratory center neurons may cause … **elevated body temperature may cause an increase in respiratory response
- stopped here on 04/11/11.
- started here on 04/12/11.
[edit] Cardiovascular response to exercise
- The cardiovascular system responds to exercise by increasing blood delivery to the muscle in three ways: vasodilation, elevated heart rate, and elevated strove volume.
- These changes can increase muscle blood flow 25-fold.
- Elevated blood supply helps to deliver nutrients and remove waste at the necessary elevated rate.
- Recall that cardiac output is a function of heart rate and stroke volume.
- As heart rate and stroke volume both increase, cardiac output increases, also.
- Heart rate increases linearly but stroke volume plateaus.
- Stroke volume may plateau because of the mechanical limitations of filling time and ejection time.
- With long term training, the heart can adapt to reach even higher heart rates and larger stroke volumes.
- Note that there is a linear relationship between cardiac output, VO2, and workload.
- Recall that VO2 is a measure of how much oxygen is used.
**However, at VO2 max, the relationship...
[edit] Blood flow distribution during rest and exercise
- There are blood flow changes throughout the body during exercise; in general, the heart and skeletal muscle are given more blood, the internal organs are given less blood, and the brain is held steady.
- Brain continues to receive the same amount of blood.
- Cardiac blood flow increases 4-fold.
- Skeletal muscle blood flow increases nearly 20-fold.
- The total cardiac output can increase nearly 5-fold.
[edit] Relationship between CO, VO2, and work
- These three variables that describe the delivery of oxygen, the use of oxygen, and the work of the skeletal muscle tissue are linearly related.
*Note that as one reaches VO2 max, oxygen consumption plateaus. **As mentioned before, work performed beyond one's VO2 max is fueled by anaerobic energy sources.
[edit] Relationship between SV and HR
- Recall that cardiac output is a function of heart rate and stroke volume.
- Heart rate and stroke volume are not increased linearly as the demand for oxygen increases.
- Recall that stroke volume plateaus before heart rate; that is, the heart rate is elevated relatively slower than the stroke volume.
- On a graph, this means that stroke volume plays a more significant role in the early phase of increased cardiac output (CO) than does heart rate.
- At the latest phases of increasing cardiac output, the elevation of the heart rate will play a more prominent role in generating increased CO (cardiac output).
[edit] SV, HR, and CO at varying levels of training
- Cardiac output of athletes and non-athletes are compared in resting and maximum effort conditions.
- Training directly affects the stroke volume, directly affects the oxygen demand of tissue, and indirectly affects the heart rate.
- Training elevates the stroke volume of the heart and decreases the oxygen demand of the body.
- Therefore, as stroke volume (even resting stroke volume) goes up (causing CO to go up) and oxygen demand (even resting oxygen demand) goes down (decreasing the CO demand), the heart rate will reach lower levels in athletes as it need not contribute as much tot he CO.
- Similarly, as SV is elevated (and therefore CO is elevated) and oxygen demand is lowered (and therefore CO demand is decreased), the heart rate will maintain lower exercise levels in athletes.
- Training causes a spread in the CO at rest and maximum effort conditions; that is, training causes decreased CO required for rest and an increased ability to generate high CO at maximum effort.
- The spread of CO is primarily due to an increase in SV and a less-significant decrease in HR (at both resting and maximum effort conditions).
[edit] Body temperature during exercise
- Recall that the body is only about 20% efficient at converting food energy to work, the rest is lost as heat.
- In exercise dates, about 75% of metabolic energy burned is lost as heat.
- During exercise, the hypothalamus may set a new (regulated) set point.
- Note that this is a regulated set point which means that it is not pathological because it will be reset when the offending stimuli (exercise) is removed.
- A new set point can be demonstrated by the elevation of rectal temperature with elevated oxygen use (that is, exercise).
- Note that an elevated hypothalamic set point reduces stress on thermoregulatory mechanisms (think sweating and cutaneous blood flow).
- Recall that one thermoregulatory mechanism is the shifting of blood flow to cutaneous regions as a vent for heat carried by the circulatory system.
- During exercise, the body maintains blood flow to skeletal muscle in spite of a shift toward cutaneous circulation.
[edit] Exercise hyperthermia
- Recall that hyperthermia is the excessive storage of heat in the body.
- During exercise, heat production elevates rapidly but heat dissipation mechanisms lag behind.
- This lag of heat loss is the reason that core body temperatures are elevated in the initial stages of exercise.
- As heat dissipation mechanisms catch up to heat generation, a new equilibrium is met and heat is not stored.
[edit] Effect of training on various cardiovascular parameters
- As mentioned before, training causes an increase in SV (and therefore an increase in CO) but does not cause an increase in HR.
- Training also causes a slight increase in oxygen extraction ability of muscle tissue (and therefore a larger A-V PO2 gradient).
[edit] Enzyme adaptation during endurance exercise training
- Long term endurance training can lead to enzyme efficiency gains in the citric acid cycle enzymes, and the converstion of glycogen phosphorylase B to A.
- Recall that glycogen phosphorylase breaks glycogen down into glucose.
- Recall that glycogen phosphorylase B is inactive and glycogen phosphorylase A is the active form.
- So to be more efficient at converting GP-B to GP-A is to be more efficient at converting glycogen into glucose.
- Other benefits from long term endurance training include:
- increased number of capillaries
- elevated maximum oxygen uptake
- increased size of muscle fibers
- Note that these benefits develop over 24 weeks of training and degrade in just 6 weeks lacking training.
[edit] Training changes muscle fiber type
- Recall that two adaptations to endurance training are increased capillary production and changes in muscle fiber type; these changes are mediated by phosphatases and kinases.
- Endurance training activates various phosphatases and kinases which act as transcriptional regulators.
- Contractile protein genes important for determining the fiber type are elevated.
- Mitochondrial genes important for mitochondrial biogenesis are elevated.
- Angiogenic growth factor genes are elevated.
Do we need to know any of the specifics from the images?
[edit] Exercise increases insulin sensitivity
- The section header says it all: daily exercise increases sensitivity to insulin.
[edit] Additional benefits of daily exercise
- Increased HDL, decreased LDL.
- Increased bone mineral density; improved coordination.
- Increased immune function.
- Need long term study evidence.
- Healthier pregnancy:
- Prevents excess weight gain in mother.
- Prevents excess weight gain in fetus.
- Prevents gestational diabetes.
- stopped here on 04/12/11.