Renal regulation of acid-base balance
From Iusmphysiology
- started here on 03/30/11.
Contents |
[edit] Renal regulation of acid-base balance
[edit] Objectives
- Describe the three processes involved in urinary acidification: reabsorption of filtered bicarbonate, formation of titratable acid, and excretion of ammonia.
- Explain why most of the hydrogen ions secreted by the renal tubules are not excreted. Explain why excretion of titratable acid and ammonia (as NH4+) adds new bicarbonate to the blood. Be able to calculate net acid excretion from measurements of urinary ammonia, titratable acid, and bicarbonate excretion.
- Discuss the factors that influence renal secretion and excretion of hydrogen ions.
- Describe the renal compensation for each kind of acid-base disturbance.
[edit] Renal acid excretion
- There are two main acids excreted by the kidney: ammonia (NH4+) and titratable acids (TA)
- In this context, titratable can be defined as "able to give a proton." NH4+ is not titratable because it will not give up a proton below a 9.2 pH.
- There is one main base excreted by the kidney: bicarbonate (HCO3-)
- Therefore, the net acid secretion by the kidney is the acids - the base:
- Renal acid secretion = TAs + NH4 - HCO3
- Normal acid secretion = 70 = 24 + 48 - 2 (mEq / day)
- The kidney can adjust acid secretion over a wide range
- Note that excreting acid is equivalent to adding new bicarbonate to the blood.
- Note that the amount of free H+ in the urine is very small.
- Acid excretion pathology:
- Acid secretion is elevated in diabetes mellitus:
- Diabetes mellitus renal acid secretion = 700 mEq / day = 200 (mEa TAs) + 500 (mEq NH4+) - 0 (mEq HCO3-)
- Acid secretion is most often elevated when consuming mixed meat / vegetable diets.
- Vegetarians excrete less acid.
- Acid secretion is elevated in diabetes mellitus:
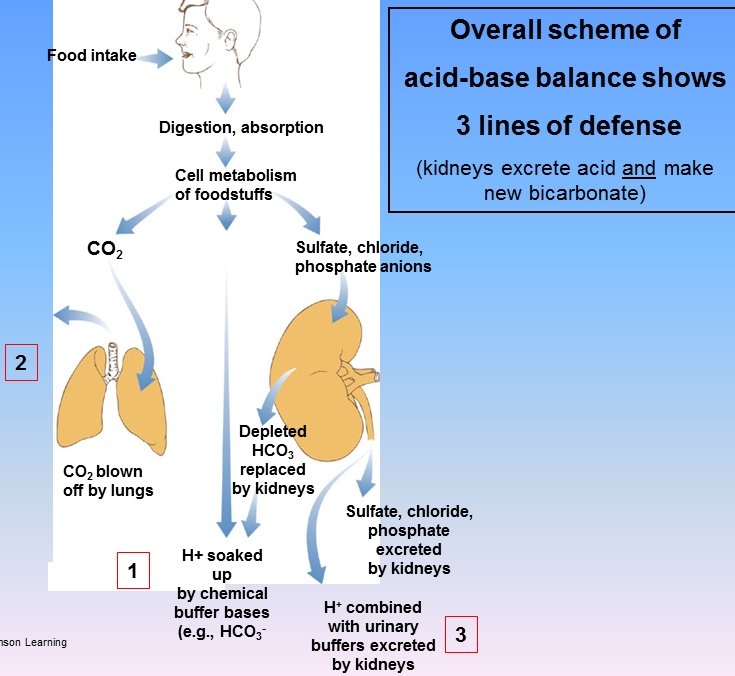
[edit] Acid-base regulation overview
- Recall from the Acid-base balance lecture that there are three regulation mechanisms: chemical buffering, pulmonary compensation, and renal compensation.
- We will now discuss the kidney's function in acid-base balance more fully.
- Recall that we previously discussed the kidney's ability to secrete H+ and / or HCO3- to rebalance the pH.
- The kidney is also capable of generating novel HCO3 and secreting titratable acids made of sulfates, chlorides, and phosphates.
- There are three processes involved in acidifying the urine:
- reabsorption of filtered bicarbonate (the more that is reabsorbed, the more acidified the urine)
- formation of titratable acid (to bind H+ cations)
- excretion of ammonia
- The production of TAs and the secretion of NH4 / NH3 results in novel bicarbonate added to the blood to replace bicarb consumed in buffering against increased acids.
[edit] Reabsorption of filtered bicarbonate
- Normally, 99.9% of filtered bicarbonate is reabsorbed by the nephron.
- When plasma HCO3 is low, there is 0 excretion.
- Note, however, that there is a threshold at which the flow rate (and therefore the amount of filtered HCO3) is so high that it cannot all be reabsorbed.
- So, this first step in urine acidification is pretty constant: the HCO3 population of base in the urine is almost always, almost completely removed and does not increase the plasma HCO3- level.

[edit] Mechanism for reabsorption of filtered bicarbonate
- As with so many things, bicarbonate is reabsorbed using the Na gradient.
- Bicarbonate from the filtrate is reabsorbed using two Na exchangers, one on the apical membrane and another on the basolateral membrane.
- Recall that HCO3- requires a transporter to cross the membrane but CO2 can diffuse across.
- Recall that in RBCs we use CA (carbonic anyhdrase) to convert HCO3- to CO2 so it can diffuse over the membrane.
- Recall that in RBCs we use a HCO3-Cl exchanger to move HCO3 in and out of the cell.
- So, in order to facilitate HCO3- reabsorption we convert it to CO2:
- Recall: H+ + HCO3- <-> H2CO3 <-(CA)-> H20 + CO2
- Filtered HCO3- exists as HCO3- in the filtrate; so we need to provide H+ to get the reaction to head toward CO2.
- A Na-H exchanger on the apical membrane reabsorbs Na and moves H+ into the filtrate.
- HCO3- + H+ -> H2CO3 -(CA)-> CO2 + H20
- CO2 enters the tubule cell.
- CO2 + H20 (both in the cell) -(CA)-> H2CO3 -> H+ + HCO3
- A Na-HCO3 cotransporter on the basolateral surface moves Na and HCO3 into the plasma.
- The aforementioned, apical Na-H exchanger moves H+ into the filtrate (to facilitate another conversion of HCO3 into CO2).

[edit] Formation of titratable acid
- Recall that the type A intercalated cells of the collecting duct secrete H+.
- Recall that H+ can readily cross back into the lining cells / interstitial fluid.
- In order to trap H+ in the filtrate, tubular cells of the nephron secrete the conjugate base of titratable acids (that is, anions that can accept another H+).
Where in the nephron does TA secretion occur?
- So, as TA secretion increases, the pH of the filtrate (urine) decreases.
- The pH of filtrate decreases as it passes along the nephron.

[edit] Mechanism for formation of titratable acid
- Note that formation of titratable acid generates NEW bicarbonate for the blood.
- Recall the Na-H exchanger on the apical surface of proximal tubule cells that was used to reabsorb HCO3-.
- Recall the Na-HCO3- cotransporter on the basolateral surface of proximal tubule cells that was used to reabsorb HCO3-.
- The same source of H (the apical Na-H) provides H+ to protonate filtered TA-salts (like HPO4-2Na) to titratable acids (like H2PO4-1Na).
- The exchange of Na for H (Na moves into the cell, H+ moves into the filtrate) requires an intracellular supply of H+.
- CA provides the H+ by combining CO2 and H20 to generate H2CO3 and then H+ and HCO3-.
- As the CA-produced H+ is moved into the filtrate in exchange for Na, the CA-produced HCO3- is moved into the blood along with Na (via the aforementioned Na-HCO3 cotransporter).
- Note that production of titratable acids uses CA and thus generates NEW bicarbonate for the plasma.

[edit] Excretion of ammonia
- First, note that when we say "ammonia" we mean both ammonium ion (NH4+) and the free base NH3.
- Recall that ammonium ion and ammonia free base live in equilibrium: NH4+ <-> NH3+ + H+
- Recall that pH can be calculated by the Henderson-Hasselbach equation if the pKa is known for an conjugate acid / base pair.
- In this case, the pKa of NH4+ / NH3+ is 9.0.
- pH = pKa + log([A-]/[HA])
- pH = pka + log([NH3]/[NH4])
- Normally, urine has a pH around 7 (though it can vary from 4.4 to 8).
- 7.0 = 9.0 + log([NH3]/[NH4])
- -2 = log([NH3]/[NH4])
- antiLog(-2) = [NH3] / [NH4]
- 10-2 = 1/100 so the ratio of NH3 to NH4 is 1:100.
- That is NH4 >>> NH3.
- So there is very little free H+ in the urine!
- Ammonia secretion by the nephron accounts for 2/3 of the H+ secreted by the kidney.
- So it is an important part of the kidney's acid-base regulation response.
- Ammonia is produced by proximal tubule cells from amino acid metabolism, especially glutamine.
- Recall that the goal is to reduce acid and increase bicarbonate.
- Note that while titratable acid production can generate new bicarbonate, it requires titratable salts (like HPO4-2Na, HSO4-Na) which are of limited supply in the filtrate.
- Therefore acid secretion by NH4/NH3 secretion is the primary method secreting lots of acid and producing lots of HCO3.
- Ammonia synthesis can be increased over a series of days and is a life saving adaptation.
[edit] Mechanism for excretion of ammonia
- Recall that we can help balance pH by secreting acid and that NH4/NH3 (ammonia) molecules are the primary acid secreted in the nephron proximal tubule.
- We will look at this process as two steps: secreting ammonia acid and generating new HCO3.
- Secreting ammonia acid:
- Recall that glutamine is the primary source of nitrogen for generating ammonia for secretion.
- Glutamine can be converted to 2 NH4 molecules by intracellular enzymes of the tubule cells.
- Here we introduce a third surface membrane: the Na-NH4 exchanger: the apical Na-NH4 exchanger moves Na from the filtrate into the cell and NH4 from the cell into the filtrate.
- Simple enough: convert glutamine into two NH4 molecules and use the Na-NH4 exchanger to secrete NH4 into the filtrate.
- Generating new HCO3 for the plasma:
- Recall that we can generate HCO3 if we can find some place to dump the H+ generated by the CA reaction.
- Recall that glutamine can be converted to alpha-ketoglutarate which--with the addition of hydrogens--can be converted to glucose.
- So with alpha-ketoglutarate (from glutamate) as an H+ acceptor (and because glucose will be happy to move out of the cell into the plasma) we can drive the CA reaction and generate HCO3.
- Once the CA reaction has generated HCO3, it can follow Na into the plasma via the same, basolateral Na-HCO3 cotransporter as was used in HCO3 reabsorption and titratable acid production.

[edit] Most H+ secretion occurs in the proximal tubule
- Recall that most of the NH4/NH3 secreted by the nephron occurs in the proximal tubule.
- Recall that most HCO3 is reabsorbed in the proximal tubule.
- There is little change in the filtrate pH in the proximal tubule because:
- most of the secreted acid reacts with HCO3 to form H2CO3 and
- the proximal tubule has a leaky epithelium through which hydrogen ions and HCO3- can pass
- The collecting duct is the site of the largest blood-urine pH gradients.
- This makes sense because it has a tight epithelium that does not allow the passage of water, H+, or HCO3-.
- Recall that type A intercalated cells actively secrete H+ into the filtrate to combat acidosis.
- Recall that type B intercalated cells actively secrete HCO3- into the filtrate to combat alkalosis.
- Highest pH blood-urine gradient is 7.4 to 4.5.
- What is the increase in [H+] over this gradient?
- 7.4 - 4.5 = 2.9
- So 102.9</sub> ~= 1000; so the urine has 1000-fold higher concentration of H+.
[edit] Factors that affect H+ secretion and excretion at the kidney
- Intracellular pH
**....
- Arterial PCO2
**Arterial PCO2 must be maintained such that CA can't function too quickly or PCO2 levels would rise too high.
- Carbonic anhydrase activity
**CA has an inherent maximum functionality; this limits the amount of HCO3 that can be produced for release into the plasma.
- Note that acetazolamide inhibits carbonic anhydrase and can thus be used as a diuretic as decreased production of intracellular H+ (from the CA reaction) means less H+ for the apical Na-H+ exchanger which means less Na reabsorption which means less water reabsorption.
- Sodium reabsorption
- Increased Na reabsorption occurs by exchange with H+ ions (into the filtrate) which means that increased Na reabsorption leads to increased acid secretion.
- Plasma potassium concentration
- If K is low, a K+-H+ exchanger moves H+ from plasma into the tubular cells and K+ from the tubular cells into the plasma.
**Rise of H+ in the tubular cell means that more H+ will be secreted.
- Aldosterone
- Elevated plasma aldosterone leads to increased Na+ reabsorption and increased apical H+ ATPase activity (which secretes H+).
**This causes increased H+ secretion....
- Availability of buffers
- As buffer availability increases, less acid needs to be secreted and less HCO3 needs to be produced.

[edit] Timeline of acid-base compensation mechanisms
- Recall that the three acid-base compensation mechanisms have differing time frames:
- chemical buffering is very fast (seconds)
- pulmonary compensation is fast (minutes to hours)
- renal compensation is slow (hours to days)

- stopped here on 03/30/11.
