Potassium, Pi, Ca, Mg balance
From Iusmphysiology
- started here on 03/29/11.
[edit] K, Pi, Ca, and Mg Balance
[edit] Objectives
- Discuss the amount of potassium in the body and its distribution.
- Define hypokalemia and hyperkalemia.
- Indicate how the following factors affect the distribution of potassium between intracellular and extracellular fluids: Na+/K+-ATPase activity, plasma pH, insulin, epinephrine, plasma osmolality, tissue trauma, infection, and hemolysis.
- Identify the sites of K+ reabsorption and secretion along the nephron and collecting duct system.
- Draw a cell model for K+ secretion by a cortical collecting duct principal cell.
- Explain how the following affect potassium excretion: dietary potassium intake, mineralocorticoids (aldosterone), acid-base disturbances, excretion of poorly reabsorbed anions, and sodium excretion (tubule fluid flow rate).
- Explain how the kidneys keep maintain phosphate and calcium balance.
- Indicate the important sites of reabsorption of phosphate and calcium along the nephron.
- State the effects of parathyroid hormone (PTH) on tubular reabsorption of phosphate, tubular reabsorption of calcium, renal synthesis of 1,25-dihydroxy vitamin D3, and bone resorption.
- Identify the major site of magnesium reabsorption along the nephron.
[edit] Renal potassium regulation
- Note that potassium input is from diet and output is via feces and urine.
- Note that potassium movement within compartments is regulated: ECF, intercellular fluid, bone / connective tissue, trancellular fluid (CSF, etc.).
[edit] The importance of Potassium
- Potassium is important for several physiological reasons.
- Potassium affects the volume of cell as it is the primary osmolyte that gets moved across cellular membranes.
- Potassium affects excitability as it is the dominant ion in determining the transmembrane potential.
- Potassium affects pH (acid-base) balance.
- Potassium affects cell metabolism as it is involved in tissue growth and repair.
- In a healthy male, 89% of the body's K is intracellular, 8% is in bone / cartilage, 2% is in the ECF, and 1% is transcellular (CSF, etc.).
- A normal plasma K is 3.5-5 mEq / L; anything above or below is hyper- / hyper-kalemia.
- Note that hyperkalemia reaching 7 mEq / L is dangerous and 10-12 mEq / L of potassium is usually fatal via cardiac arrhythmia / arrest.
[edit] Movement of K between ICF and ECF
- There are many reasons K moves out of cells: low ECF pH, digitalis, O2 lack, hyperosmolality, hemolysis, infection, ischemia, and trauma.
What's the difference between O2 lack and ischemia?
- There are several reasons K moves in to cells: elevated ECF pH, insulin, epinephrine.
- Clinically, hyperkalemia can be treated with sodium-bicarbonate (IV) which will push K into cells by alkalinizing the ECF (that is, elevating the ECF pH).
- Hyperkalemia can also be treated with insulin + glucose (IV) as increased insulin drives K in to cells.
[edit] Kidneys filter, reabsorb, secrete, and excrete K
- Recall that we filter 180 L of plasma each day.
- Potassium (K) is normally maintained at 4 mEq / L.
- For comparison, recall that Na is maintained at 140 mEq / L.
- About 90 mEq of potassium are excreted each day (about 12.5% of the potassium filtered).
- Recall that only 0.9% of the Na filtered each day is excreted.
- Potassium (K) is normally maintained at 4 mEq / L.
- The kidneys excrete 90% of our daily intake of potassium.
[edit] K reabsorption within the nephron
- As with Na, most of the secretion / regulation of K occurs at the collecting duct.
- Just as only about 10-15% of the water of filtrate makes it to the collecting duct, only about 5% of the K of filtrate makes it to the collecting duct.
- 70% of filtered K is reabsorbed in the PCT (like Na).
- 25% of filtered K is reabsorbed in the LoH.
- Recall that the descending branch is permeable to water and solute in both directions.
- Recall that the ascending branch is impermeable to water and actively reabsorbs Na (actively pumps Na out of the filtrate).
- When plasma K levels are low, the collecting duct and continue reabsorption until only 1% of filtered K remains in the filtrate.
- When plasma K levels are high, the collecting duct can secrete K such that the filtrate contains 150% as much K as was filtered.
- Recall that normal physiological state results in 15% of the filtered load being excreted in the urine.
[edit] K secretion at the collecting duct
- The main site for regulated K secretion is the principal cells of the collecting duct.
- Recall that the principal cells of the collecting duct use ENaC and Na-K ATPase to regulate Na reabsorption.
- In a similar manner, principal cells use their basolateral Na-K ATPase to drive up the intracellular K concentration and their apical K channels to allow the K to flow into the filtrate via electrochemical gradient.
- So, just as the Na-K ATPase moves Na and K in opposite directions, the channels on the membrane allow them to flow opposite directions (out of and into the filtrate, respectively).
- So, principal cells respond to elevated K via their basolateral Na-K ATPase and apical K channel.

[edit] K reabsorption at the collecting duct
- The main site for regulated K reabsorption is the type A intercalated cells of the collecting duct.
- Recall that type A and type B intercalated cells of the collecting duct regulate blood pH by secreting H+ and reabsorbing HCO3- (type A cells) or secreting HCO3- and reabsorbin H+ (type B cells).
- Recall that intercalated cells (both types) generate H+ and HCO3- via the protein AE1.
- Recall, too, that intercalated cells secrete / reabsorb H+ by way of an apical H+ / K+ ATPase: that is, they burn ATP to exchange H+ / K+ at the apical membrane.
- Type A intercalated cells secrete H+ to balance an acidotic plasma and do so by reabsorbing K+ from the filtrate.
- Therefore type A intercalated cells are the primary location of K reabsorption in the nephron.
- type A intercalated cells respond to aldosterone-induced K loss:
- When mineralocorticoids are found in excess (think aldosteronism), Na is heavily reabsorbed at the collecting duct (think ENaC and Na-K ATPase) at the expense of K.
- Therefore type A intercalated cells attempt to compensate for the aldosterone-triggered K loss by secreting acid in exchange for K.
- This is the mechanism for renal alkalosis compensation.
- Recall that the body can compensate elevated pH (alkalosis) via the lungs (hypoventilation, breath off less CO2) or the kidneys (secrete H+ into the filtrate).
[edit] Factors that increase K excretion
- There are several factors that increase K excretion; some are pro-reabsorption effects and some are anti-reabsorption effects.
[edit] Increased dietary K intake increases K excretion
- As dietary K increases, plasma K will increase.
- As plasma K increases both the adrenal glomerulosa cells and the principal cells of the collecting duct respond.
- Glomerulosa cells of the adrenal glands increase production of mineralocorticoids in response to elevated plasma K.
- Increased amounts of mineralocorticoids leads to increased exchange of Na for K at the collecting duct and therefore increased K excretion.
*Principal cells of the collecting duct will How do principal cells respond to elevated plasma K levels?
[edit] Increased mineralocorticoids increases K excretion
- Recall that mineralocorticoids generally have a delay of hours before their response because they are steroids that bind intracellular receptors that act as gene expression regulators.
- As mineralocorticoids increase (think aldosterone), more Na is exchanged for K at the collecting duct (ENaC / Na-K ATPase).
- Exchange is increased because the number relevant surface proteins is increased (ENaC, Na-K ATPase, and K channels) and the production of ATP is increased.
- As more K is pumped into the principal cell more will flow into the filtrate by way of electrochemical gradient and K channels
[edit] Increased blood pH increases K excretion
- As the blood pH rises, the type A intercalated cells (think "aaaaah! too much aaaaacid!") respond by pumping H+ out of the blood into the filtrate in exchange for K+.
- As H+ is pumped into the filtrate (from the type A intercalated cell cytoplasm), K+ is brought into the cytoplasm and then moves into the blood via electrochemical gradient and K channels.
- Note that in acute acidosis the converse occurs: high levels of H+ in the ECF leads type B cells secreting H+ in exchange for K+ from the filtrate and thus K+ excretion is decreased.
[edit] Increased concentration of poorly absorbed anions increases K excretion
- Recall that an electrochemical gradient occurs over the apical membrane of the epithelial cells that line the nephron tract and that the gradient must be (at least somewhat) charge balanced.
- That is, the electrochemical gradient over the membrane cannot be super large.
- Therefore, if many negatively charged ions exist in the filtrate there must be some positively charged ions present also.
- As the concentration of anions increases, the movement of K out of principal cells increases (that is, K excretion increases).
[edit] Increased Na+ excretion increases K excretion
- Recall that elevated Na excretion means there is increased flow of filtrate (water follows Na, so if the amount of Na is elevated so is the volume of filtrate).
- When filtrate volume increases, so does the flow rate (more volume through the same area).
- As the flow rate increases there is an increase in electrochemical pull of K out of the principal cells and into the filtrate.
- Furthermore, for Na excretion to increase, the collecting duct principal cells must not reabsorb all the Na--but, all the apparatus for reabsorbing Na (think ENaC, Na-K ATPase) that does exist on the cell membranes will probably be working at maximum capacity (even if there is very little of it because there is very little aldosterone signaling) so K will be sacrificed for the Na (via the aforementioned Na-K ATPase).
[edit] Na deprivation does not lead to K excretion
- Now that we know that K is sacrificed in order to reabsorb Na, we might think that a pt deprived of Na would end up getting rid of lots of K (that is, filtrate would be low in Na, jg apparatus detects, signals granular cells to release renin, renin generates angiotensin, angiotensin stimulates the glomerulosa layer of the adrenal cortex, aldosterone released, ENaC and Na-K ATPase added to membranes of principal cells and K would be pumped into the filtrate in order to reabsorb all the Na out of the filtrate possible).
- However, there is a second mechanism that balances this this reaction and yields no extra loss in Na.
- (In the case of Na deprivation) filtrate Na would be low, jg apparatus detects, signals renin release, angiotensins are at high levels, both the afferent and efferent arterioles constrict, GFR decreases, Na reabsorption in the PCT increases, flux through collecting duct decreases, and decreased electrochemical pull of K out of the principal cells occurs.
I thought the PCT's reabsorption of Na was constant? (Na reabsorption lecture)
- In short: the aldosterone response increases K excretion but the GFR (decrease) response decreases K excretion.
[edit] Potassium excretion deficiency
- Potassium excretion is deficient in Addison's disease and renal failure.
- Recall that aldosterone tells the collecting duct to express ENaC and Na-K ATPase.
- Recall that as Na is reabsorbed via ENaC, it is driven by the Na-K ATPase-generated Na gradient and therefore Na is retained at the expense of K lost to the filtrate.
- Recall that Addison's disease is a deficiency in aldosterone production.
- In renal failure, there is deficient response to aldosterone.
- Poor aldosterone production / response means poor expression of ENaC / Na-K ATPase and poor reabsorption of Na / loss of K.
- Chronic renal failure is defined as GFR < 20 ml / min.
- Note that deficiency in excretion can lead to hyperkalemia.
[edit] Potassium excretion excess
- Potassium excretion is elevated by hyperaldosteronism and (most) diuretics:
- When aldosterone is elevated (as in hyperaldosteronism), lots of ENaC is put on the apical membrane of principal cells of the collecting duct and lots of Na-K ATPase activity causes lots of K moved into the cell and thus loss of K into the filtrate via electrochemical pull.
- Similarly, most diuretics decrease Na reabsorption upstream of the collecting duct and thus there is more Na in the filtrate to be reabsorbed by ENaC / Na-K ATPase at the collecting duct and thus lots of K movement into the principal cells and subsequently lots of K loss to the filtrate due to electrochemical gradient.
- Note that excessive potassium excretion will lead to hypokalemia.
[edit] Renal regulation of Pi
[edit] The importance of Pi
- Pi is an important buffer in the plasma and urine; recall that Pi usually has one or two H bound which it can release to buffer bases.
- A normal plasma Pi level is 1 mM.
- There is usually a 4:1 ratio of HPO4 to H2PO4.
- Most of the Pi in the body is stored in the bone, but some of the bone is remodeled daily such that Pi is released and added back allow for an exchange of bone Pi for dietary Pi.
- Usually about 2/3 of our dietary Pi is absorbed and an equal amount is excreted at the kidney.
[edit] Kidneys filter, reabsorb, and excrete Pi
- Phosphate is easily filtered by the glomerulus.
- Most of the filtered Pi is reabsorbed at the proximal tubule (and very little is reabsorbed beyond the proximal tubule).
- There is a Na-Pi cotransporter on the apical (brush border) membrane of the proximal tubule cells.
- Recall that Tm is the maximum rate at which a transporter can move molecules across a membrane.
- The amount of filtered Pi often exceeds the Na-Pi cotransporter's Tm and thus Pi is generally lost to the filtrate.
- Recall that this isn't all bad, though, because Pi is a nice buffer molecule for the urine.
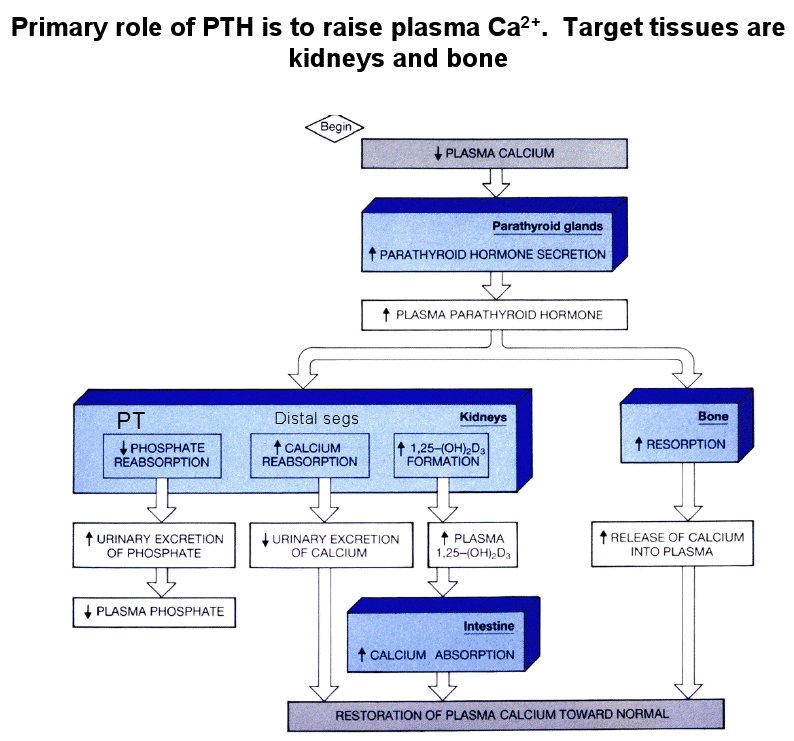
- PTH is the primary regulating molecule / mechanism for Pi
- Recall that PTH is released by the parathyroid to increase Ca levels.
- PTH causes rapid endocytosis of the Na-Pi cotransporter at the proximal tubule and thus increased Pi loss.
[edit] Renal failure leads to bone disease, precipitates, and renal transplant
- During renal failure, there is poor PTH response by the proximal tubule reabsorption cells and therefore an increase in plasma Pi called hyperphosphatemia.
- Recall that a decrease in PTH response means less Na-Pi transporter removed from the surface; that is, decreased PTH response leads to increased plasma Pi levels.
- Renal failure also leads to decreased tubular mass (cells are dying).
- Both increased plasma Pi and decreased VitD activation lead to less VitD activation at the kidney.
- Recall that VitD is made at the skin and activated at the liver and kidneys.
- Decreased VitD activation leads to decreased Ca reabsorption at the gut.
- Recall that VitD is a necessary transcription factor for some protein that helps transport Ca across the enteric cell cytoplasm.
- Decreased Ca reabsorption at the gut means less Plasma Ca.
- Less plasma calcium leads to more PTH release.
- Elevated PTH leads to Ca and Pi release at the bone.
- Ca and Pi release at the bone leads to bone loss, Ca-Pi precipitation, and brittle blood vessels.
Q: In "renal failure" how do we know what fails? For example we say "renal failure leads to hyperphosphatemia" but the etiology of all these failures in regulation are not explained. It could be the PTH signaling or the apical membrane protein that moves Pi or.... A: We define renal failure (at least chronic) as a GFR < 20 (mL / hour). Therefore, "failure" or deficiencies in regulation occur because there is such a low volume of plasma being filtered that the regulatory mechanisms of the renal tubule cells (think acid secretion, Na reabsorption, etc.) cannot be effective.
[edit] Renal regulation of Ca
- Normally, only 20% of dietary Ca is absorbed.
- This can be increased by VitD.
- Like Pi, most of the body's Ca is in the bone but some of the bone is remodeled each day providing an exchange of Ca between compartments.
- Also like Pi, in a stable situation the amount of Ca absorbed by the gut is matched by the amount secreted by the kidney.
- A normal plasma Ca is about 2.5 mM.
- Recall that normal Pi plasma level was 1 mM.
- Unlike Pi, about 50% of the plasma Ca is bound to plasma protein and therefore not filtered.
[edit] The importance of Ca
- Ca is a very tightly regulated plasma ion.
- Ca is important for cellular depolarization and as a cofactor for many cellular reactions.
[edit] Kidneys filter, reabsorb, and excrete Ca
- Recall that 50% of the plasma Ca is bound to plasma protein and therefore not filtered.
- Ca reabsorption in the nephron uses an apical Ca channel and basolateral active transport.
- Filtered Ca is reabsorbed primarily by the PCT (60%) and the LoH (30%).
- Regulated reabsorption of Ca occurs in the ascending limb, the DCT and the connecting tubule.
- Here, PTH increases the reabsorption of Ca (mechanism not given).
[edit] Renal regulation of Mg
- 99% of Mg is in bone and cells.
- Mg is neglected but second only to K in abundance.
- A normal plasma Mg is 0.8-1.0 mM
- Recall that a normal plasma Ca is 2.5 mM.
- Recall that a normal plasma Pi is 1.0 mM.
- 30% of plasma Mg is bound to protein.
[edit] The importance of Mg
[edit] Kidneys filter, reabsorb, secrete, and excrete Mg
- Recall that 30% of plasma Mg is protein-bound and therefore not filtered.
- The kidney reabsorbs 90% of the filtered Mg.
- Reabsorption of Mg occurs primarily in the loop of Henle (60% of the renal reabsoprtion)
- Mg reabsorption occurs by paracellular movement, driven by the positive potential of the filtrate that wants to push positive ions like Mg2+ out.
- Recall that "paracellular movement" means the Mg moves from filtrate to interstitial fluid by going around the junctions of the cells.
- Similar movement is achieved by other positive ions, too: Na+, K+, Ca++, and NH4+.
- stopped here on 03/29/11.
