P's quiz 3 study guide
From Iusmphysiology
(Difference between revisions)
75.207.16.204 (Talk)
(Created page with '*started here on 04/04/11. ==Sexual differentiation and the HPG Axis== ===Learning objectives=== *Prof will take exam questions from objectives. ===Sexual differentiation=== …')
Newer edit →
(Created page with '*started here on 04/04/11. ==Sexual differentiation and the HPG Axis== ===Learning objectives=== *Prof will take exam questions from objectives. ===Sexual differentiation=== …')
Newer edit →
Revision as of 12:49, 7 April 2011
- started here on 04/04/11.
Sexual differentiation and the HPG Axis
Learning objectives
- Prof will take exam questions from objectives.
Sexual differentiation
- Genetics is determined at fertilization.
- XY = male
- XX = female
- The sperm has either an X or a Y and donates it to the X-containing ovum.
- There are many levels of sexual differentiation:
- establishing the genetic sex
- differentiation of the gonads
- differentiation of the internal reproductive organs
- differentiation of the external genitalia
- gender role
- gender identity
Differentiation of the gonads
- As an embryo develops, the gonads become the source of gender hormones:
- In males, the gonads become the testes and provide testosterone and dihydrotestosterone.
- In females, the gonads become the ovaries and provide estrogen.
- The gonads take their developmental cues from their genotype as to how it should develop and what hormones it should produce.
- An XY gonad has a Y chromosome with the Sex-determining region Y (SRY).
- SRY is also called testis determining factor (TDF).
- SRY is the master switch that causes differentiation to head toward male.
- SRY encodes a transcription factor that is part of the high mobility group (HMG) family.
SRY and PAR on the Y chromosome
- The PAR (psudoautosomal region) of the Y chromosome is a well conserved area that allows the Y chromosome to pair with the X chromosome for cell division.
- PAR is at the very distal area of the short arm of the Y chromosome.
- SRY is located just proximal to the PAR and is considered part of the sex determining region.
- Two diseases are associated with SRY:
- SRY defects lead to XY females; Swyer syndrome.
- Translocation of the SRY region from the Y chromosome to the X chromosome yields XX males; XX male syndrome.
Differentiation of the internal genital ducts
- Initially, embryos initially have a set of undifferentiated gonads and both Wolffian ducts and Mullerian ducts.
- The ducts become the transporters of sperm or egg.
- Wolffian ducts mature into the epididymis and vas deferens.
- Mullerian ducts mature into the oviduct, uterus, and upper part of the vagina.
- Based on the genotype of the gonads (that is, the presence or absence of SRY), the gonads will begin to express hormones.
- Testes produce AMH (anti-Mullerian hormone), testosterone, and dht (dihydrotestosterone).
- AMH causes involution of the Mullerian ducts and testosterone causes proliferation of the Wolffian ducts.
- Ovaries produce no hormones embryonically.
- A lack of hormones allows Wolffian ducts to involute and causes Mullerian ducts to proliferate.
- Testes produce AMH (anti-Mullerian hormone), testosterone, and dht (dihydrotestosterone).
- The presence of hormones from the gonads determines the differentiation of the internal genitalia.
- If SRY is present:
- AMH, test, and testosterone are produced by the developing gonads
- Anti-Mullerian hormone (AMH) is responsible for degeneration of the female-associated Mullerian ducts in males
- We say that the Mullerian ducts involute; involute: "rolled inwards spirally" per [www.biology.lsu.edu/heydrjay/ThomasSay/terms.html LSU Biology]
- Gonads differentiate into testes.
- If SRY is not present:
- No hormones are produced by the developing gonads
- The Wolffian ducts atrophy.
- Gonads differentiate into ovaries.
- Note that female seems to be the default gender.
Swyer syndrome
- Recall that Swyer syndrome results from a SRY defect in an XY patient.
- Swyer syndrome is considered a type of hypogonadism because the expected male gonads did not develop.
- Swyer syndrome is considered a "pure" gonadal dysgenesis because there is no chromosomal defect; that is, they have a normal karyotype.
- Gonads are underdeveloped and are often referred to as "streaks".
- Not that though the gonads do not develop correctly in Swyer syndrome, the internal and external genitalia do develop normally.
- Note, however, that puberty does not occur normally so external genitalia do not mature through puberty.
- Patients with Swyer syndrome are often treated with estrogen and progesterone replacement therapy.
Klinefelter's syndrome
- Klinefelter's syndrome results from a 47 XXY genotype.
- XXY genotype results in poorly developed testicles.
- Underdeveloped testicles can result in non-masculine features and pro-feminine features:
- Non-masculine: poor beard growth, poor chest hair growth, frontal hair growth (lack of frontal balding), small testicular size
- Pro-feminine features: narrow shoulders, wide hips, breast development, female-like pubic hair growth
- 1:1000 males has Klinefelter's syndrome
Differentiation of external genitalia
- Like gonads and ducts (internal genitalia), the external genitalia begin in a bipotent state from which they can develop into either male or female external genitalia.
- External genitalia are signaled to develop by the presence or absence of androgens--particularly DHT.
- Male external genitalia develop in the presence of DHT.
- Female external genitalia develop in the absence of DHT.
Listen for how much anatomy we need to know.
- Said he won't ask specific details; just wants us to know that the pre-anatomy has bipotential.
- One exam question from everything previous to this comment.
Gender role
- Gender role is the gender presented by an individual to society.
- Can be independent from anatomy and chromosomes.
- Gender role can be expressed through name, clothing, physical appearance, family role, occupation, and behavior.
No exam questions on this.
Gender identity
- Gender identity is the internal conviction of one's own gender.
- We do not currently understand all the factors and complexity of gender identity.
- There is an interesting, intimate relationship between nature and nurture as it relates to development of role identity.
- Think prenatal androgen exposure, family beliefs, appearance of the genitalia, and medical / surgical experiences.
No exam questions on this.
- One exam question from everything after this comment.
Key concepts of the HPG axis
- The HPG axis is the hypothalamus-(anterior)pituitary-gonad axis.
- Note that the HPG axis also includes some activity from the cortical regions of the brain (the higher-function centers of the brain).
- Some examples of higher brain centers that affect the hypothalamus are the visual, olfactory, pineal and stress centers.
- The hypothalamus contributes to the HPG axis by releasing GnRH.
- GnRH binds to receptors on the gonadotropes of the anterior pituitary.
- The gonadotropes of the anterior pituitary contribute to the HPG axis by releasing leutinizing hormone (LH) and follicle stimulating hormone (FSH).
- The gonads contribute to the HPG axis by secreting sex steroids and peptide hormones.
- The gonads also release inhibin which feeds back on the anterior pituitary to reduce LH and FSH release.
- The gonads are also the site of germ cell production and maturation.
- Testosterone and estrogen from the gonads feed back on the anterior pituitary and the hypothalamus to reduce LH / FSH and GnRH release, respectively.

HPG axis in males
- In males, the hypothalamus releases GnRH to affect gonadotropes of the anterior pituitary.
- Upon GnRH signaling, gonadotropes of the anterior pituitary release LH and FSH to affect the testicles.
- LH and FSH negatively feedback on the hypothalamus, too.
- Upon LH / FSH signaling, the leydig and sertoli cells of the testicles release testosterone and inhibin.
- Testosterone triggers spermatogenesis and negatively feeds back on the anterior pit and hypothalamus.
- Inhibin inhibits the anterior pituitary.
- Note that testosterone is bound by ABP (androgen binding protein) in the blood.
http://www.uptodate.com/contents/images/ENDO/5463/HPG_axis_PI.jpg?title=HPG+axis+PI
HPG axis in females
- In females, the hypothalamus releases GnRH to affect gonadotropes of the anterior pituitary.
- Upon GnRH signaling, gonadotropes of the anterior pituitary release LH and FSH to affect the ovaries.
- Note that LH / FSH don't negatively feed back on the hypothalamus like they do in the male.
- Upon LH / FSH signaling, granulosa cells of the ovaries release estradiol, progesterone, inhibin, and activin.
- Estradiol and progesterone go on to affect target cells.
- Estradiole and progesterone have opposite feedback effects on the anterior pit and hypothalamus depending on the phase: positive feedback in the follicular phase and negative feedback in the luteal phase.
- This makes sense because females need to make and mature oocytes on a cycle each month.
- Activin increases FSH production and release and systemically increases proliferation.
- Inhibin decreases FSH production and release and systemically decreases proliferation
- Estradiol and progesterone go on to affect target cells.

Higher centers
- The HPG axis is affected by stress, sight, smell, and emotion.
- These emotions can generate inhibitory or stimulatory signals.
No exam questions on this.
Neurotransmitters that affect the HPG axis
- There are LOTS of NTs that affect the HPG axis: norepinephrine, dopamine, epinephrine, acetylcholine, endorphins / opioids, neuropeptide Y, leptin, serotonin, cholecystokinin, GABA-major inhibitory NT.
Hypothalamus
- The hypothalamus releases GnRH at 70-90 minute intervals; we call this autorythmicity.
- GnRH is a chromosome 8, 10mer peptide with a very short half-life--around 3 minutes.
- The cells that secrete GnRH are neurons located in the arcuate nucleus of the medial basal hypothalamus (MBH).
Immortalized GnRH secreting neurons
What is the point of this slide?
Pituitary Gonadotropins
- FSH and LH are released by gonadotrophs of the anterior pituitary.
- FSH and LH are alpha-beta in structure; alpha is identical but beta is unique.
- This won't be tested.
- Gonadotrophs are stimulated (to release FSH and LH) and inhibited by GnRH and gonad hormones, respectively.
Hypothalamus and Pituitary anatomy
- An illustration highlighting the point that gonadotropes reside in the anterior pituitary.

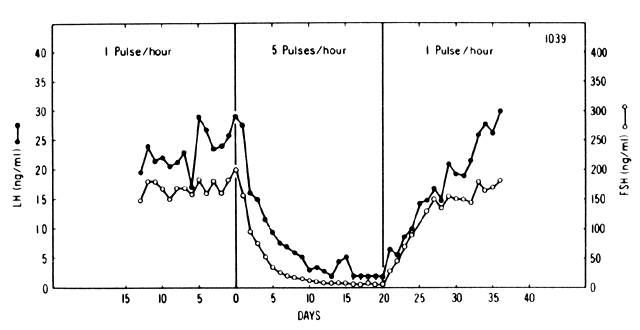
Pulsatile versus continuous GnRH
- When you override the pulsatile release of GnRH by infusing lots continuously, LH / FSH drops to low levels.
- So we can see that it is important that GnRH must be released pulsatile to get normal release of LH / FSH.
Control of the onset of puberty
- Puberty: the period of transition between juvenile state and adulthood, during which secondary sex characteristics appear and fertility is acquired.
- We say that puberty occurs when the HPG axis matures, but we don't know the catalyst for puberty.
- We do know that the onset of puberty is affected by many factors, including: genetics, nutrition, body weight, skeleton maturation (affects estrogen levels), altitude.
- We suspect that psychosocial and environmental factors (like environmental estrogen exposure) also play a role in determining the onset of puberty.
Mini-puberty of infancy in males
- In males, during the first month of life, there is a period of adult-like HPG axis activation.
- That is, a period where testosterone levels are equal to those of adult males.
- The function of this mini-puberty in boys is unknown.
- There is no appreciable change in physical characteristics caused by these high levels of testosterone.
Testosterone throughout the lifespan
- Testosterone is seen during the first and second trimesters of pregnancy, primarily.
- Then test is expresed during the mini puberty and begins to ramp up again during the 10-17 years (puberty).
- Test expression remains constant through most of adult life and then begins to fade in old age.
- http://books.google.com/books?id=9gvBlktAT6YC&lpg=PA1&ots=L23cN_r6NM&dq=kaefer%20m%20Mechanisms%20manifestations%20and%20management&lr&pg=PA256#v=onepage&q=testosterone&f=false
Changes in the HPG axis during puberty
- During puberty, the HPG axis is "maturing".
- Decreased sensitivity of GnRH-releasing neurons (hypothalamus) to negative feedback (from the gonad hormones) causes an increase in pulsatile GnRH release.
- Increased sensitivity of gonadotrophs (anterior pit) to GnRH causes an increase in LH / FSH secretion.
- Increased sensitivity of gonads to LH / FSH causes increased gonadal steroid production.
- stopped here on 04/04/11.
- started here on 04/05/11.
Kisspeptin and GPR54 at the Hypothalamus
- Neurons of the hypothalamus is stimulated to release GnRH when kisspeptin binds GPR54.
- GPR54 is a 7-transmembrane protein: bind extracellular signal and then transduce the signal via the cytoplasmic tail.
- Kisspeptin is one of several peptides encoded by the Kiss-1 gene.
- When GPR54-Kisspeptin signaling is interrupted, hypogonadotropic hypogonadism results from reduced LH / FSH signaling.
Characteristics of normal puberty
- There are four aspects to a normal puberty phase.
- Sexondary sexual characteristics develop: things that are not directly related to making babies (facial hair, breast enlargement, et cetera).
- Somatic growth spurt occurs
- Fertility is acquired
- Physiological changes occur
Puberty terminology
- Adrenarche: onset of adrenal and androgen production
- Precedes puberty by 2-3 years
- Occurs around 7-8 years old
- Thelarche: onset of breast bud development
- Estrogen causes thelarche
- Greek / latin: thel- nipple, female
- Pubarche: onset of pubic hair growth
- Estrogen or testosterone causes pubarche.
- Menarche: onset of menstral flow
- Average age of menarche onset in the US is 12.8 years old
Secondary sexual development
- Gonadarche: rise in gonadal sex steroids as a result of the HPG axis re-activation (recall that it was active in pre-natal development).
- Adrenarche: rise in adrenal androgens independent of gonadal sex steroid production
- We know that estrogens and androgens cause some of the changes seen in puberty because aberrant exposure to estrogens and androgens causes aberrant changes.
Physical effects of sex steroids
- Estrogena 'and androgens cause growth acceleration, skeletal maturation, and genital changes.
- Estrogens cause breast development in both boys and girls.
- Androgens cause body hair, body odor, and also causes acne in both boys and girls.
Puberty in girls
- Age of onset between 7.5 years to 13 years; average age of onset is 10.25.
- The first sign of puberty is breast buds in 70% of cases.
- Another common first sign is pubic hair.
- A second sign of puberty usually follows within 6 months.
- The peak growing time for women usually occurs 1.3 years before menarche.
- Average growth during this growth period is 9 inches.
- Menarche usually occurs 2.25 years after the onset of puberty.
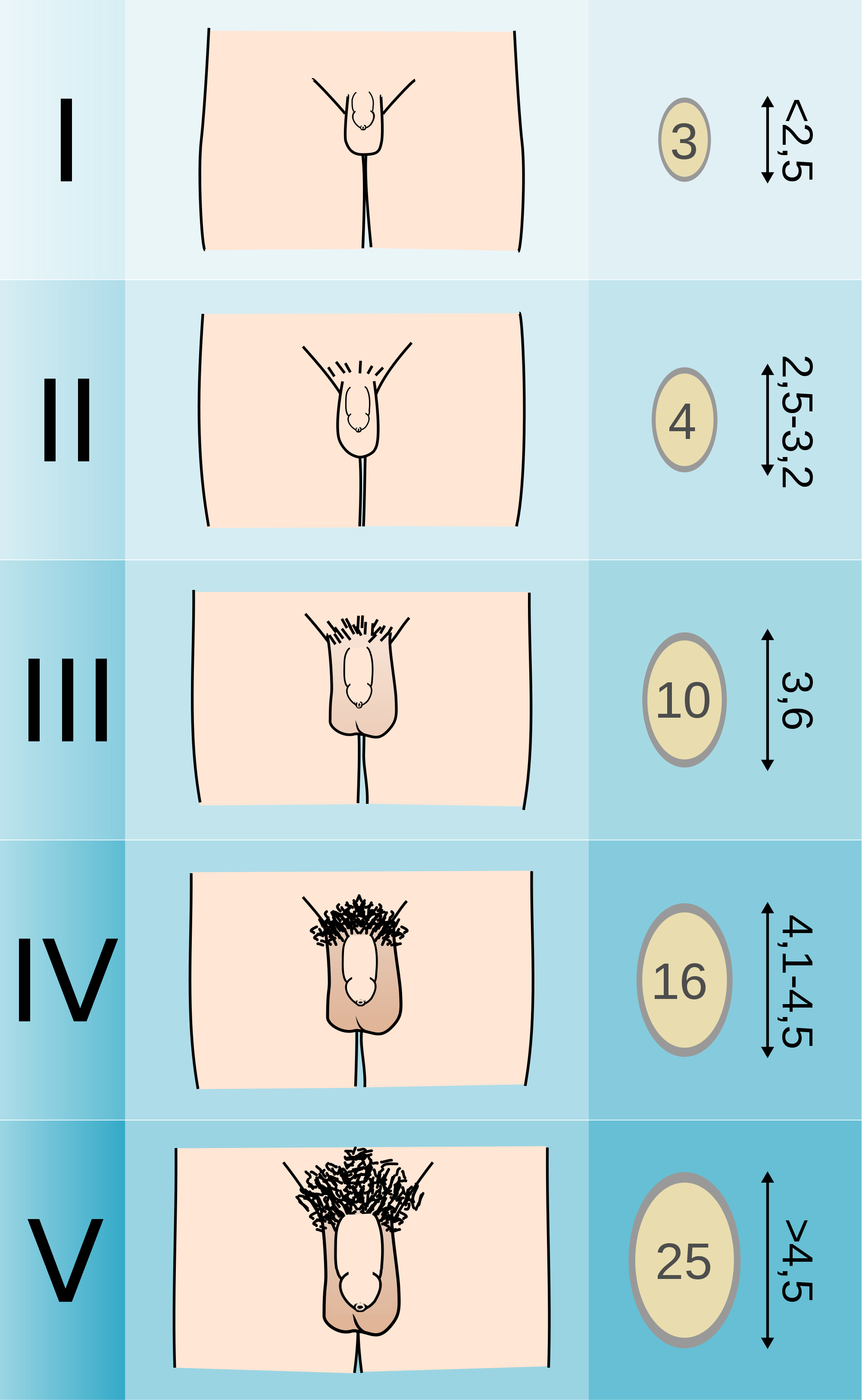
Puberty in boys
- Age of onset between 9 years to 14 years; average age of onset is 12.25.
- The first sign of puberty in boys is testicular enlargement.
- One can measure the testicular volume as an indicator of enlargement.
- The peak growing time for men is usually 2 years later than in girls.
- Boys usually gain around 11 inches during pubertal growth spurt.
Puberty comparison: boys and girls
- Boys start and end later.
- Girls start earlier and proceed more rapidly through puberty.

Abnormal puberty
- There are lots of causes of abnormal puberty--some are normal variation and some are pathological.
- Any junction of the HPG axis can be involved.
- The treatment depends on the etiology.
Precocious puberty
- Precocious puberty defined as "secondary sexual development occurring in girls before the age of 7.5 / 8 (AA, Hispanic / caucasians) or in boys before the age of 9".
- There are 3 types of precocious puberty: normal variants, central, and peripheral.
- Normal variants resulting in precocious puberty can occur by way of premature thelarch (recall that thel refers to breast in greek or latin) or premature adrenarche (adrenal or adrogen production).
- Central precocious puberty arises from defects of the HPG axis.
- Peripheral precocious puberty arises from an ectopic (non HPG) source of sex steroids.
Central precocious puberty
- Most cases of precocious puberty are central precocious puberty (having to do with the HPG axis).
- Central pp (precocious puberty) results in a normal sequence of events just at an earlier time; that is, it looks just like puberty but occurs earlier in the patient's life.
- Central pp is much more common in females.
- Central pp: females > males
- Central pp's etiology is usually idiopathic.
- CNS injuries can increase the risk for central pp. (Blows to the head, spinal injuries, etc.)
- Secondary sexual development occurs gradually.
- Somatic growth (which is a normal part of puberty) also starts early, is accelerated, and is then arrested relatively early (even for precocious puberty) and thus results in short stature.
Causes of precocious puberty
- Recall that precocious puberty is ultimately the early release of sex hormones.
- Tumors or hyperactivity of the pituitary or hypothalamus can cause early release of the sex hormones.
- 60% of pp boys have an identified brain abnormality.
- Most girls under 4 with pp have an identified brain abnormality.
- 80% of girls with pp do not have an identified brain abnormality.
- Pseudoprecocious puberty results from a tumor of the adrenal / testes / ovary that releases sex hormones.
- In pseudoprecocious puberty, the gonads do not develop early (because they are not getting the required LH / FSH signaling) but the aberrant levels of sex hormones will cause secondary sexual development.
Precocious puberty: Symptoms and diagnosis
- Male and female S&S: underarm / pubic hair growth, body odor change, acne, early growth, early arrest of growth, short stature,
- Male S&S: facial hair growth, penis lengthening, appearance becomes masculine
- Female S&S: menstruation, breast development
- Recall that one difference between true and pseudo- precocious puberty is the development or lack of development in the gonads, respectively.
- In true precocious puberty, the gonads develop because there are elevated levels of LH and FSH.
- In pseudoprecocious puberty, the gonads do not develop because there are not elevated levels of LH and FSH.
- Diagnostics include measuring blood hormone levels and taking x-rays of the hand and wrists for estimates of bone development.
- CT, MRI, and ultrasound are also used to look for adrenal / hypothalamic / pituitary tumors and development of the adrenals and gonads.
A GPR54-activating mutation
- Recall that the GPR54 receptor resides on the neurons of the hypothalamus (in the MBN) and is activated by kisspeptin.
- This research identified a mutation in the GPR54 receptor that activated the receptor and caused central precociouis puberty.
- Recall that turning on GPR54 increases GnRH which increases LH / FSH at the pit which causes development of the gonads.
- Specifically, the mutation caused a decrease in receptor desensitization such that the receptor transduced an intracellular signal for a longer period of time than a wild-type receptor.
- This decreased densensitization caused increased signaling through the GnRH releasing neurons and increases GnRH release.
- This image shows the amount of phosphorylated ERK as a measure of pathway activation.
- In the disease state, there is more phosphorylated (activated) ERK present.
Peripheral precocious puberty
- Recall that peripheral precocious puberty occurs when sexual development is induced by sex steroids that do not originate from the HPG axis.
- Peripheral precocious puberty is rare and can be heritable or not.
- The non-HPG source of steroids can be endogenous or exogenous.
- Peripheral precocious puberty often demonstrates heterogeneity:
- there is often acute onset,
- there is often linear growth acceleration that results in tall stature and advanced bone age (upon xray diagnostics on the hand and wrist),
- there are many different classes of steroids to which children can be exposed,
- the duration of exposure to steroids can be quite variable.
McCune-Albright syndrome, a form of peripheral precocious puberty
- One cause of peripheral precocious puberty has been named: McCune-Albright syndrome results from an activating mutation of a G protein expressed in endocrine tissues.
- The G protein's Gs-alpha subunit is mutated into a higher activity state causing increased cAMP.
- Elevated cAMP from an over-active G protein causes hyperfunction of endocrine tissues.
- McCune-Albright is characterized by a triad of symptoms: pp, cafe au lait, and fibrous bone dysplasia.
- Large ovarian cysts are also seen in girls.
- McCune-Albright precocious puberty is an example of a somatic mutation in a mosaic distribution.
Why is it mosaic? Because it mutates during development? Yes per wikipedia
Delayed puberty
- We consider puberty delayed if there is no female onset by 13 or male onset by 14.
- We also consider pubertal development slower than one Tanner stage per year delayed puberty.
- Delayed puberty can either be "normal variant" or pathologic.
- Normal variant delayed puberty shows similar delay in both somatic growth and sexual development and often occurs with a family history of "late bloomers".
- Pathologic delayed puberty can be congenital or acquired and may be caused by a problem at any level in the HPG axis.
Conclusion
- The HPG axis is a highly integrated system with inhibitory and stimulatory modulators.
- Though we don't know the trigger for puberty, we do know the predictable series of events that normally occur.
- There are many different etiologies for abnormal puberty, many of which affect the HPG axis.
- stopped here on 04/05/11.
- started here on 04/06/11.
Male reproductive physiology
Learning objectives
- Recognize the structure and functions of the male reproduction organs
- Understand the process of spermatogenesis
- Understand steroidogenesis
- Understand disorders of male reproduction
Anatomy
- The creamaster muscle and the pampiniform plexus help regulate the temperature of the testes.
- The cremasteric muscle can pull the testes up toward the abdomen for increased heat.
- The pampiniform plexus of vessels provides lots of blood flow and lots of heat.
How much more anatomy do I need to know from this slide?
Characteristics of the testes
- The testes have two functions: spermatogenesis and steroidogenesis.
- The seminiferous tubules are the site of spermatogenesis.
- The seminiferous tubules are very small tubules that radiate through the testicle from the rete testis.
- Two cell populations comprise the seminiferous tubule: germ cells and Sertoli cells.
- Germ cells generate new sperm.
- Sertoli cells play a supporting role to germ cells.
- Leydig cells produce testosterone.
- Leydig cells are also called "interstitial cells of the testes" because they reside between the seminiferous tubules.

- Note the epididymis, seminiferous tubules, lobules, and vas deferens.
Structural organization of the testes
- The cellular organization within the seminiferous tubules is focused on generating a blood-testes barrier.
- The blood-testes barrier is important to prevent immunological attack upon the developing sperm.
- To form this barrier, sertoli cell surround the germ cells that generate sperm.
- Sertoli cells span the entire width of the seminiferous tubule.
- There are two compartments in the seminiferous tubules: basal and adluminal compartments.
- The basal compartment contains the least mature sperm and is nearest the border of the tubule.
- The adluminal compartment contains the most mature sperm and is in the center of the tubule.


Sertoli cell supportive functions
- Recall that sertoli cells are supportive cells to germ cells.
- Sertoli cells are important for spermatogenesis in three ways: phagocytizing defunct germ cells, spermiation, synthesis of transferrin.
- Spermiation: "the release of the spermatozoon from the seminal epithelium into the lumen of the seminiferous tubule." per academia
- Sertoli cells provide plasmin, an enzyme that causes release of spermatozoa from the epithelium of the seminiferous tubule into the lumen.
- Recall that spermatozoa are fully mature male gametes.
- Note that transferring is important for spermatogenesis.
Sertoli cell secretory functions
- Sertoli cells have g-protein FSH receptors that drive cAMP and PKA.
- cAMP and PKA in sertoli cells have several effects, all of which generally lead to increased signaling and proliferation.
- Increased release of androgen binding protein.
- Recall that ABP carries testosterone and DHT in the blood.
- Increased release of inhibin.
- Recall that inhibin inhibits the pituitary (FSH / LH).
- Induction of P450 aromatase
- Recall that P450 aromatase converts testosterone to estradiol.
- Increased release of plasminogen activator
- Recall that plasminogen activator is a serine protease that breaks down clots.
- Increased cell proliferation
- Increased release of androgen binding protein.
Sertoli cell products
- Sertoli cells proudce three major products: ABP, Inhibin, Plasminogen activator
- Androgen binding protein:
- 90Kd, heavy and light chain
- Binds Test and DHT with high affinity
- Used for carrying androgens in the Sertoli cells and epididymis.
- Used for storing androgens in the seminiferous tubule
- Inhibit
- Provides negative feedback to the pit.
- Plasminogen activator
- Cuts plasminogen into plasmin which goes on to digest fibrin (a key structural protein in clotting).
Leydig cells
- Recall that leydig cells reside in the interstitium, between seminiferous tubules.
- Know this, it kind of makes sense that leydig cells are derived from mesenchymal cells as they are sitting in a connective tissue area.
- Leydig cells are especially focused around blood vessels.
- Leydig cells are primarily a reservoir of lipids that can be used to generate testosterone.
- Leydig cells appear foamy because of the presence of secondary lipid droplets.
- The lipid droplets are esterified cholesterols which can be used for testosterone production.
- Testosterone production requires two major steps and three locations:
- The first major step occurs in the cytoplasm: hydrolyzing the esterified cholesterol into free cholesterol.
- The second major step occurs in the mitochondria: converting cholesterol into pregnenolone.
- Note that chol->pregnenolone is the rate-limiting step.
- The third location is the endoplasmic reticulum where testosterone is finally generated.
Leydig actions
- Leydig cells have LH receptors that stimulate release of testosterone.
- Sertoli cells and Leydig cells demonstrate reciprocal hormonal communication.
- As the Leydig cells release testosterone (via LH signaling), the Sertoli cells use their P450 aromatase to convert it to estradiole (E2) and send it back to the Leydig cells.
- The function of E2 (estradiol) signaling on Leydig cells is unclear.
Additional products of the testes
- The testes also make several other products:
- Opiods
- AVP
- Oxytocin
- GnRH-like peptide
- Growth factors
- Neurotransmitters
Anything more to know from this slide?
Spermatogenesis
- Spermatogenesis is a well scheduled event so there are phases and cycles.
- The three phases are: mitosis, meiosis, and spermiogenesis
- Cycles last 65-70 days and new cycles begin every 2-3 weeks.
Germ cell mitosis and meiosis
- The cells of spermatogenesis proceed in a particularly named order through a series of specific types of divisions:
- Cell (count): primordial germ cell (1) -> spermatogonia (1) -> primary spermatocyte (32) -> secondary spermatocyte (64) -> spermatids (64) -> spermatozoa (64)
- Note that primordial germ cells, spermatogonia, and primary spermatocytes are diploid whereas secondary spermatocytes, spermatids, and spermatozoa are haploid.
- Divisions: Mitosis -> meiosis 1 -> meiosis 2.



Spermiogenesis
- Spermiogenesis (as opposed to spermatogenesis) is the specialization of the spermatid into the spermatozoa.
- Four major changes take place to form a highly specialized cell:
- Nearly all the cytoplasm is lost.
- Nuclear chromatin is condensed and altered.
- The axoneme (tail) is formed: centrioles rearrange and relocated to form a 9x2 arrangement on the cell membrane.
- the axoneme has a fibrous sheath, too.
- The acrosome is foromed: a collection of enzymes surrounds the nucleus at the opposite pole as the tail.
- There are three major sections to the completed spermatozoa:
- The head, made of the acrosome (collection of enzymes).
- The middle, made of spiral sheathes of mitochondria for energy.
- The tail--also called the axoneme--propels the cell forward by a twisting motion.
The axoneme
- Recall that the axoneme is generated from the spermatid's centriole.
- There is an intricate structure to the axoneme that is important for its function.
- At the center is the central tubule with 2 tubules.
- Radiating from the outside toward the center are the radial spokes.
- Radial spokes are connected to the exterior structure by Y links.
- Radial spokes are connected to adjacent radial spokes by bridges.
- Along the radial spokes are 9 sets of doublet tubules.
- The axoneme uses dyenein motors on the doublet tubules to generate a spiral twisting which generates movement.
Role of testosterone and FSH in spermatogenesis
- Both FSH and testosterone are required for spermatogenesis.
- Recall that Sertoli cells have receptors for both FSH and testosterone.
- Recall that testosterone is generally coming from the Leydig cells because of LH signaling.
- Recall that FSH is coming from the anterior pituitary.
- While FSH is required for initiation of spermatogenesis, testosterone is sufficient to maintain progressing spermatogenesis.
- Testosterone is required for capacitation: the process that allows spermatozoa to generate motion and occurs in the epididymis.
Seminal fluid
- Seminal fluid (semen) is the entire combination of fluids ejaculated.
- Seminal fluid contains:
- 10% sperm by volume
- seminal vesicle fluid: 75% by volume
- prostatic secretions
- bulbourethral secretions
- Seminal fluid has fructose (nutrient for spermatozoa), ascorbic acid (I think has something to do with maintaining pH once in the female UG tract), prostaglandins (increase blood flow), and fibrinolysin (cut up clots).
Expulsion of semen
- Semen expulsion is a neuromuscular reflex with two phases: emission and ejaculation.
- Emission moves sperm and the rest of the seminal fluid contributions into the urethra.
- The lumbar spine nerves control the muscular contractions of the epididymis and vas deferens.
- Ejaculation moves semen out of the urethra.
- Ejaculation requires a second spinal reflex and contraction of the bulbospongiosus muscles that surround the uretra.
- Recall from anatomy that Pointing and Shooting require Parasympathetics and Sympathetics, respectively.
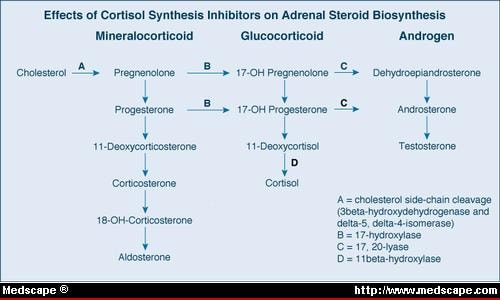
Testicular steroidogenesis
- Recall the hormone synthesis pathway of the adrenal gland:
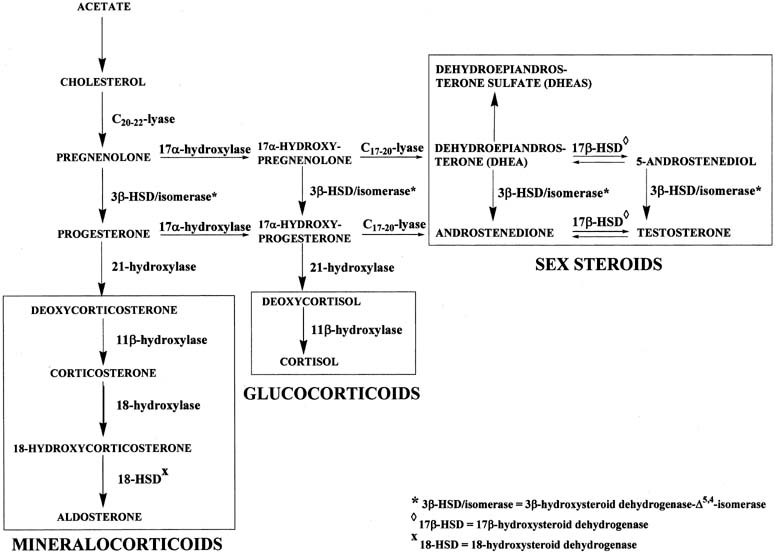
- Which of these end products is produced is determined by the predominant enzyme present.
- By genetic determination of the predominant enzyme expressed, tissues like the testes, the adrenal glands, and the ovaries can become functionally specific.
- In the testes, testosterone is the primary product generated.
- Pregnenolone is the key intermediate for the testes.
- Note that delta 5 or delta 4 can be used along the pregnenolone -> testosterone pathway.
The LH receptor
- Recall that the Leydig cells contain cholesterol stores and generate testosterone upon LH signaling.
- Leydig cells have 15K LH receptors / cell yet less than 5% need to be activated to generate a response.
- This means that Leydig cells can detect very low levels of LH.
- The LH receptor signals through a g-protein coupled receptor, cAMP, and PKA.
- Ultimately, testosterone is generated and released.
Familial male precocious puberty (FMPP)
- Familial male precocious puberty (FMPP) is also called testotoxicosis.
- Testotoxicosis is "familial" because a mutation of the LH receptor can cause this disease.
- Mutations of the LH receptor have been shown to cause excessive inactivation (and thus excessive and early release of testosterone and thus precocious puberty).
- At least 15 mutations have been identified.
- Most mutations involve ASP478.
- Clinical manifestations of FMPP include:
- precocious puberty by age 2-6
- secondary sexual development
- acne
- growth acceleration and advanced skeletal maturation
- behavior problems
- Note that female carriers of the mutated LH receptor are unaffected.
- Treatment of FMPP:
- Apply an androgen receptor blocker to inhibit the excessive and early testosterone.
- Apply an aromatase inhibitor lest the testosterones get aromatized into estrogen (via Sertoli cells as one example).
Metabolism of testosterone
- Recall that testosterone is generated by Leydig cells upon LH signaling.
- Testosterone is a steroid so it can immediately diffuse out of the producing cell into the blood.
- In the blood, most testosterone (97-98%) is bound to carrier proteins (steroid hormone binding globulin, SHBG).
- SHBG acts as a reservoir for testosterone.
- SHBG is increased by estradiol signaling on the liver.
- Plasma testosterone is generally converted to DHT or E2 (estradiol) at the target tissue:
- DHT: prostate, scrotum, penis, bone
- E2: fat, liver, CNS, skin, hair
- 17 ketosteroid: liver, kidney
- Test: testes, pituitary, muscle
- Conjugate: liver, kidney
DHT, dihydrotestosterone
- DHT is two to three times more potent than testosterone.
- DHT is critical for normal sexual differentiation.
- 5-alpha reductase converts testosterone to dihydrotestosterone (DHT).
- 5-alpha reductase is also active in hair follicles and sebaceous glands.
Estradiol
- Estradiol is generated by many tissues (including testes and the brain) from testosterone.
- Abnormal levels of E2 causes gynecomastia (breast enlargement).
*E2 levels in men rise nearly to those of women when the man goes through the follicular phase. Does he really mean this?
Actions of androgens
- Androgens have affects on physical differentiation, brain masculinization, and physiological properties.
- Physical differentiation: sexual differentiation (see Sexual differentiation, HPG axis), secondary sexual characteristic development (hair, acne, voice, growth), hair growth (and balding).
- Physiological properties: lipid levels, RBC mass
- Masculinization of the brain
- There are many androgen receptors on the brain.
- Although we know that hierarchy and copulating patterns in animals are correlated with androgens, there is no correlation between libido and androgen levels in human males.
- Females exposed to excess androgens during development (in utero) demonstrate increased "male typical" behavior.
Worldwide trends in male reproductive function
- We are currently observing trends that make us think reproductive function may be declining:
- serum testosterone levels are decreasing in the US and Europe.
- Sperm concentration has decreased by nearly half over the past 70 years.
- Semen quality differs geographically and has been shown to correlate with pesticide expsoure (less semen production upon exposure).
- There is an increased incidence of cryptorchidism, hypospadias, and testicular cancer.
- Cryptorchidism: "a condition seen in newborns whereby one or both of the male testes has not passed down into the scrotal sac." per Driscoll Chidlrens
- Hypospadias: "an abnormal condition in males in which the urethra opens on the under surface of the penis" per Princeton's wordnetweb
Disorders of male reproduction
- Half of the infertility of men is caused by endocrine disorders.
- Hypothalamic-pituitary hypogonadism results from a defect in the HPG axis: Kallman syndrome, GPR54 gene mutations, and GnRH receptor mutations.
- Recall that GPR54 receptor mutations lead to decreased GnRH release by the neurons of the hypothalamus upon kisspeptin signaling.
- Recall that GnRH receptor mutations lead to decreased LH / FSH release by the gonadotrophs in the anterior pituitary upon GnRH signaling from the hypothalamus.
- 'Primary hypogonadism occurs because of defects in the gonads: Klinefelter syndrome, testicular regression, acquired hypogonadism
- Recall that Klinefelter syndrome results from a 47XXY karyotype that causes underdeveloped testes and therefore decreased testosterone levels.
- Note that testicular regression syndrome covers "a variety of conditions in which both testes regress during fetal life. It is also known as pure gonadal dysgenesis, Sawyer's syndrome, true agonadism, testicular dysgenesis, vanishing testis and complete bilateral anorchia" per Medcyclopaedia.
- Recall that "true" gonadal dysgenesis resulted from a defect in the SRY region.
- Acquired hypogonadism can occur because of infection or abuse of anabolic steroids.
- Other endocrinopathies: hyperprolactinemia, excess androgens
Kallman syndrome
- Kallman syndrome results from the mutation of the cell adhesion gene KAL.
- KAL is ubiquitously expressed in all tissues of the body and even escapes X inactivation.
- KAL is located on the p arm of the X chromosome.
- The KAL gene generates anosmin as a protein product; anosmin is an important component of the basement membrane.
- When KAL is mutated in XY males, hypogonadotropic hypogonadims and anosmia results.
- Without functiona anosmin, GnRH secreting neurons of the olfactory placode fail to migrate to the medial basal hypothalamus (MBH).
- Improper migration leads to olfactory bulb dysgenesis and decreased GnRH release:
- In some cases, the pt cannot smell
- In most cases, the pt has poor gonad development, poor secondary sexual characteristic development, and poor puberty development.
Conclusions
- Testes produce both steroids and sperm.
- Spermatozoa are the end product of spermatogenesis and are highly specialized cells.
- Androgens affect physical appearance, the brain, and physiology.
- Disorders of male reproduction can result from endocrine and non-endocrine (Kallman Syndrome) etiologies.
- stopped here on 04/07/11.