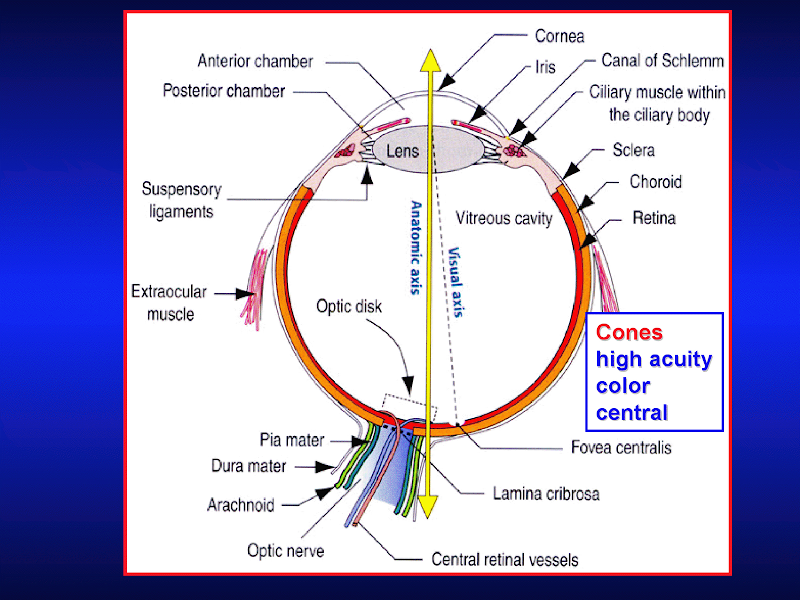
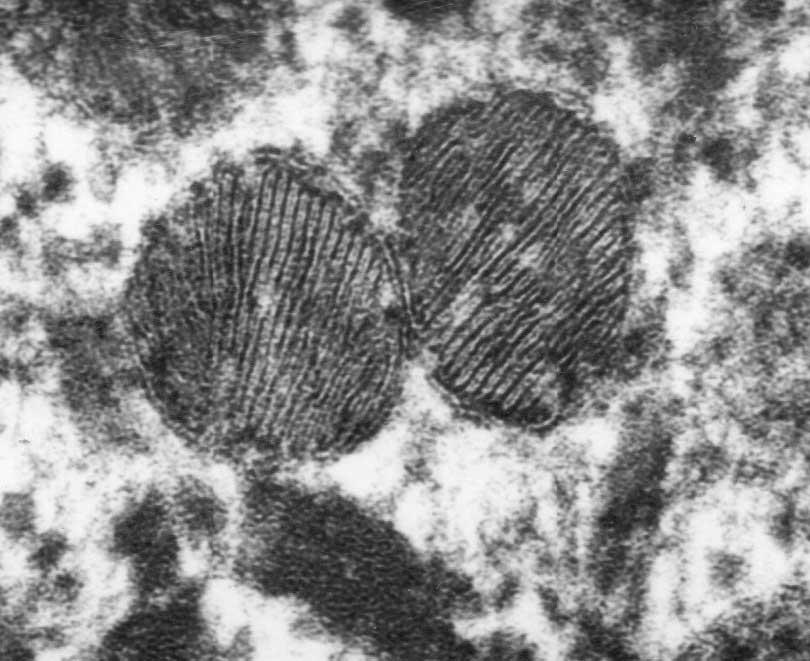
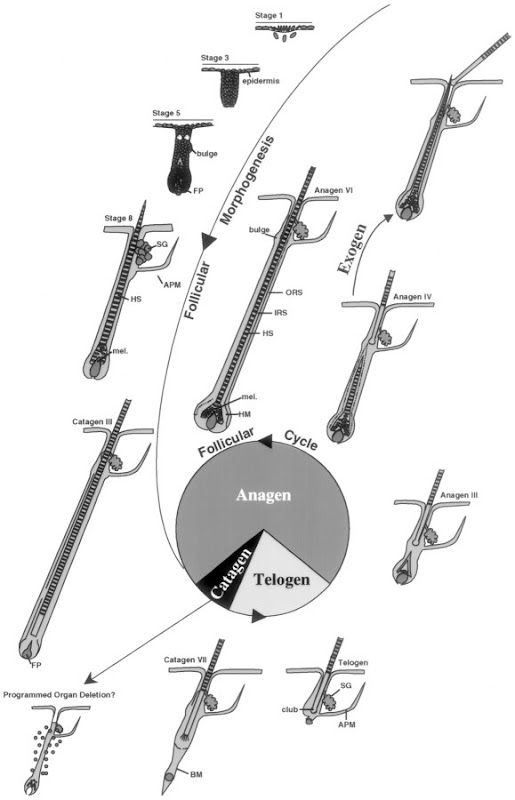
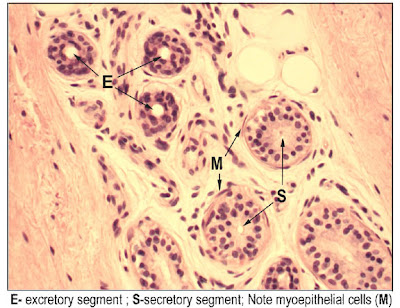
P's NBME study guide
From Iusmhistology
[edit] Contraction in detail
- If Ca+ is low, then tropomyosin will inhibit myosin to bind to thin filament.
- So ADP and Pi will be held on myosin but bind and contraction are not occurring.
- If Ca+ rises to 1 micromolar or greater, then Ca binds to TnC (troponin subunit of thin filament).
- TnI and TnT (parts of troponin) then are involved in conformational change.
- TnI binds actin.
- TnT binds tropomyosin.
- Calcium binding on troponin changes the conformation of troponin such that TnI comes up off actin which allows tropomyosin to move about 5 minutes around
the clock face of the actin.
- This allows the head of myosin to bind in on the thing filament.
- Upon binding to actin, the Pi is released from myosin. This causes a conformational change--the power stroke.
- ADP is then released placing the apparatus in a "rigor mortis" state. ATP must mind before actin is released from myosin.
[edit] Intercalated disks
- Fascia adherens
- Where thin filaments are joined together.
- A bit like zonula adherens.
- Where thin filaments joined to function as one between cells.
- Macula adherens
- Just a desmosome
- Where thick filaments pass between cells (?).
- Gap juctions
- Electrical connections.
- membranes come together very close at gap jucntions
- Don't physically hold cells together because they don't affect cytoskeleton.
- Occur along the longitudinal axis of the muscle cells, generally.
- Fascia adherens
[edit] NERVOUS SYSTEM
[edit] Astrocytes
- Most numerous glial cells in CNS
- Two types
- Fibrous:
- Long, thin processes
- Protoplasmic astrocytes:
- Found in grey matter
- Short and fat processes
- Fibrous:
- Have end feet: connect to epithelium and sit on the external surface of the CNS
- Provide physical support for neurons
- Maintain homeostasis (toxin processing, extra NT processing, etc)
- Release neurotrophic factors (regulate transuction, still unknown)
- Can be found between two neurons and may help transduce signals
- Astrocytes can interact with neurons through the neuron's spine and their own form of a spine.
- Astrocytes are increased after ischemia of the brain (cns).
- So in early ischemia, astrocytes may proliferate in order to rescue the neurons.
- When ischemia is severe enough that neurons don't survive, the astrocytes generate a type of scarring material.
[edit] Blood brain barrier
- Has four components:v Endothelial cells , basement membrane, end feet of astrocytes, pericytes
[edit] Microglia
- These are macrophages in the CNS
[edit] PERIPHERAL NERVE
[edit] Slide 9 sciatic nerve dog
[edit] Slide 17 Spinal cord ganglion, mammal
- The dorsal root sends afferent ("at" the CNS) fibers from the dorsal root ganglion to the dorsal horn of the spinal cord grey matter.
- The ventral root carries efferent ("exit" the CNS) fibers from the spinal cord to to visceral motor effectors.
- The ventral horn contains cell bodies of somatic motor neurons.
- The lateral horn houses cell bodies of autonomic motor neurons that innervate smooth muscle and glands.
- Somatic motor neuron axons run through the ventral root and bypass the DRG.
- Visceral motor neurons have cell bodies in the ventral horn; axons travel through the ventral root, bypass the dorsal root ganglion, follow nerves that lead
to peripheral ganglia.
- Note that this is a one-cell communication between the CNS (spinal cord) and the effector cell (skeletal muscle).
- The autonomic nervous system takes two cells for CNS-to-effector communication.
- Here there are preganglionic cells and post ganglionic cells with ganglia as their point of synapse.
- The cell body of the preganglionic cells is in the spinal cord.
- The cell body of the postganglionic cells is in the respective ganglia.
- There are two divisions to the autonomic nervous system: parasympathetic and sympathetic.
- The sympathetic system is characterized by short pre-ganglionic axons that synapse in ganglia that is very near to the spinal cord (think "sympathetic
chain ganglion", etc.).
- Subsequently, the post-ganglionic fibers are much longer as they run to their effector cells.
- The parasympathetic system is characterized by long pre-ganglionic fibers that run to ganglia that are far from the spinal cord.
- Subsequently, the post-ganglionic fibers of parasympathetics are short fibers that run to effector cells that are close to their ganglia.
[edit] Epithelium lecture
[edit] Tight jxn
- Tight jxns = zonula occludins
- There are several protein components:
- ZO1 and ZO2 (zonulin occludin)
- Claudin
- Occludin
[edit] The belt desomosome
- At the top of the cell, below the zonula occludin.
[edit] Hemi-desmosome
- These occur ont he base of the cells, to connect them to ECM (connective tissue).
[edit] Gap jxn
- Connexons make up these pores.
[edit] Basement membrane
- Has a lamina densa and one or two lamina rara (lamina lucida)
- BM formed by type 4 collagen.
- This type does not form fibrils.
- There are lots of glycoproteins in bm:
- laminin
- proteoglycans, too
[edit] Circulatory
[edit] Histology of vessels
- Layer closest to the blood is tunica intima.
- Outside most layer is called tunica adventitia or tunica externa.
- In between is the tuica intermedia
- Contains muscle
[edit] Capillaries
- They only have the tunica intima.
- Sometimes have pericytes with them
- Continuous capilarries:
- Have a relatively thick (though very thin) extension of cytoplasm
- Fenestrated capilarries
- Have windows = fenetre in french
- Found in kidney, GI, and endocrine glands (pit, thryroid)
- More leaky than continuous
- Fenestrated capilarries without diaphragm
- Have windows
- Found in the renal glomerulus
- More leaky than fenestrated with
- Sinusoidal capilarries
- Bone marrow, liver, lymphod tissue
- Where lots of proteins move in and out of blood
[edit] Image examples
- Pinocytotic vessles are much like the fenestra.
- Also called caveoli
- Can fuse to cause little diaphrams
- Diaphragm of fenestra
- Made of PB1 and other proteins
- PB1 forms fibrilar spokes across the fenestra.
- Tunica intima:
- In healthy tissue, all vessels contain only simple squamous epithelium called endothelium.
- large elastic artery:
- medium (muscular) artery:
- arteriole:
- capillary:
- venule:
- medium vein:
- large vein: the exception
- tunica intima of the large veins is a thicker layer as it includes connective tissue and smooth muscle.
- Tunica media:
- Manifests itself as smooth muscle in all vessles (except caps and venules where it does not exist)
- Some layers consist of additional materials
- large elastic artery: has elastic layers (lamini)
- medium (muscular) artery: has elastic fibers not layers
- arteriole: not much eleastic fiber
- capillary: no tunica media at all
- venule: no tunica media at all
- medium vein:
- large vein:
- Tunica adventitia:
- On the outside
- Found on all larger vessels
- More on veins (that is, larger walls) than on arteries
- large elastic artery:
- medium (muscular) artery:
- arteriole:
- capillary:
- venule:
- medium vein:
- large vein: the exception
- has connective tissue
- has longitudinal bundles of smooth muscle
[edit] Connective Tissue
- Imagine the oldest person you know...naked. That's connective tissue deficiency.
[edit] Twin study
- This is called elastosis
- Collagena ndn elastic fibers are losing their strength.
[edit] Ground substance
- Made up of glycosaminoglycans and ...
- Glycosamino glycans:
- Put into aggregates via hylauronic acid.
- Glycosaminoglycans (GAGs)
- There are multiple flavors.
- Syndecan (integral membrane protein)
- Versican (
- Aggrecan
[edit] Another componetn of ground substance
- Laminin (not lamin)
- Binding sites for just about everything in the BM
- Type IV collagen
- Heparin sulfate
- Integrins
- Collagens, sulfates, lipids, etc.
- Binding sites for just about everything in the BM
- Fibronectin
- Again, a velcro
- many binding sites
[edit] Integrins
- Dimeric proteins with alpha an dbeta subunit.
- Connect via talon on the inside of the membrane
- Signaling goes inward and outward.
- This is how the cell knows where it is and who its neighbors are and what it should be doing.
[edit] Collagen
- Gly-X-Y repeats are really impt.
- Forms a left handed helix.
- However, collagen is always found in triple helix turn that has a right handed turn.
[edit] Fibrillar collagens
- 1, 2, and 3 for fibrils
- only visible in em
- 1 and 3 also form collagen fibers
- Fibrils spontaneously line up in head to tail conformation
- In an EM, this is a big hint that you're looking at some type of collagen.
[edit] Type 4 collagen
- Interuptions allow for flexible kinks.
- Head molecule allows for interaction with other collagen fibers.
- Thisis the foundation of the BM.
- Perlecans help hold the mesh together.
[edit] Collagen synthesis
- Requires vit c; survvy
- Glycosylation in ER
[edit] Elastic fibers
[edit] State 1
- Oxytalan fibers
- Resist stretch
- When oxytalon fibers are lost, skin just hangs.
[edit] Stage 2
- Elaunin
- At this stage, deposits of elastin are irregularlly place thorught he scaffold of oxytalan.
- Elastin is a globular protein with glycine and proline in it.
[edit] Stage 3
- As secretion (by fb) continues, the elastin fibers crosslink (giving stretchability) and organizes in a regular way between fibrils.
- This gives skin the smooth and supple look.
[edit] Loose connective tissue versus dense
- Loose connetive:
- Fibers in all directions
- Dense irregular
- Not as many cells
- No specific orientation
- Dense regular tissue
- Tendons and ligaments
- All fibers in one direction
[edit] Connective Cells
[edit] Red marrow
- Stroma
- Where cells reside
- Hematopoietic cords
- site of blood cell formation, contains CFUs
- Sinusoidal capillaries
- Blind ends
[edit] Erythroblasts
- Erythropoietin (made by kidney) stimulates macrophage
[edit] RBC membrane
- Spectrin and anchorin are key in discoid shape.
[edit] Neuts
- 60-70 % of leukocytes
- Number of sengments of nucleus is indicative of cell's age.
- Young neuts = band cells (horse-shoe shaped nuc)
- Have p-selectin ligands for slowing and tumbling on endothelium.
[edit] Eosinophils
- 2-4% of the
- 2-5 nuclei sections
[edit] Basophils
- < 1% of leukocytes
- Irregular nuc
- Usually bilobed
- Hard to sse because granules stain so well
- Proteins:
- Heparin, histamine
- mediate inflammation
- Act much like mast cells
[edit] Agranulocytes
[edit] Thrombocytes
- There is an open system (an invagination of the membrane)
- For nutrient uptake
- Tubular system
- Microfilaments that wrap around the cell
- Release things from cells
[edit] Lymphoid organs
[edit] Cell type localization in lymphoid organs
[edit] Thymus
- Hassall's corpuscles consist of epithelial reticular cells in concentric circles with keratin filaments filling the cells.
[edit] Activity of the cortex and medulla
- The cortex is isolated from circulation which is important so that T cells don't get away before they have been selected.
- There are three structures that generate this Thymocyte-Blood barrier:
- Continuous capillaries with tight junctions and a basal lamina; this keeps T cells from moving away aberantly.
- Reticulocytes (type I) that are bound to one another with desomsomes to help stop
- Perivascular connective tissue occupied by macrphages
- The boy in the bubble
- SCID pt, no B cell, T cell, or NK
- Mutation in the gamma chain of some receptor.
- Tonsils:
- Tonsils are considered partially encapsulated
- The capsule is only found on the side that faces the oral cavity.
- This is useful for keeping microorganisms out.
- Palatine tonsils, pharyngeal tonsils, and lingual tonsils.
- Tonsils are considered partially encapsulated
[edit] Encapsulated lymphoid organ organization
[edit] Spleen
[edit] =Blood flow in the spleen
- The splenic artery branches to form trabecular arteries which give rise to central arteries which follow the trabeculae.
- The central arteries are surrounded by white pulp and the T cells within.
- After traveling past the white pulp, the arteries split again into penicular vessels.
- Penicular vessels are a specialized set of vessels that carry blood into the red pulp.
- Penicular vessels pass blood into sheathed capillaries.
- Sheathed capillaries are surrounded by macrophages, not by endothelial cells.
- At the red pulp, blood splits it's flow: closed circulation or open circulation.
- Closed circulation:
- This type of circulation is called closed because the blood is continuously bound by endothelial cells of the vessel walls.
- In closed circulation, the penicullar arterioles and capillaries connect to the sinusoids.
- In closed circulation, macrophages reach between the endothelial cells to detect and destroy old erythrocytes.
- Open circulation:
- Open circulation allows blood to flow into the stroma of the splenic cords.
- The penicular arterioles are open-ended and thus let the blood flow directly into the splenic cords.
- In the stroma, macrophages destroy aged / abnormal erythrocytes or any erythrocyitic chunks floating about.
- Aged RBCs are targeted because their membrane is not flexible enough to let them get through the basement membrane of the sinusoids thorugh which they
must pass if they want back into the circulation.
- Intact RBCs leave the stroma via trabecular veins and the splenic vein.
[edit] RESPIRATORY SYSTEM
-Bowman's gland= serous glands; secretions contain odorant-binding protein (OBP) which binds odorant molecules, carries them to receptors on specialized
cilia
[edit] Conducting region
-Vestibule= contains vibrissae (nasal hairs);
Phacynx = connects the nasal cavity to the larynx; segments are the nasophacynx and oropharynx
Trachea= from base oflarynx. to start ofbronchial tree; hyaline cartilage rings in 'C' shaped pattern; perichondrium attached to trachealis muscle (smooth
muscle) which serves to contract lumen Bronchial Tree = from bronchi to terminal bronchioles; progressive transition to smaller diameter
Bronchioles = no glands or cartilage but still has smooth muscle; some goblet cells; progressive transition from ciliated pseudostrati:fied columnar
epithelium to ciliated simple columnar epithelium to cuboidal; final portion is called terminal bronchiole
Clara cells = secrete alveolar fluid which serves as the aqueous phase ofsurfactant
[edit] Respiratory region
Alveolar ducts = contains smooth muscle and both elastic and reticular fibers
[edit] Cells ofthe alveolar system
-Type I {squamous alveolar cells)= flat cells that line majority ofalveolar surface; provide a minimum thickness barrier for gas exchange; contain both
desmosomes and occluding junctions
-Type II (great alveolar or 'niche' cells) =cuboidal cells located in comers ofalveoli (groups of 2-3 cells); contain lamellar bodies which secrete pulmonary
surfactant (acts to lower surface tension such that less pressure is required to keep alveoli open); can differentiate to replace injured type I cells
Neonatal respiratory distress syndrome (RDS) = surfactant deficiency in premature infants (production begins at 35th week ofgestation); collapse of alveolar
walls; treated with corticosteroids which stimulates synthesis of surfactant or artificial surfactant.
-Alveolar macrophage (dust cell)= in alveolar septum and alveolar surfaces; remove degraded surfactant; either migrate up the bronchial tree or remain in the
alveolar wall for life
-Endothelial cells= Thin, no fenestrations; express angiotensin-converting enzyme (important in control ofblood volume/pressure)
Fused basement membranes
[edit] Blood-air barrier
Three components: 1) Surface lining and cytoplasm ofthe alveolar cells 2) Fused basement membrane ofthe alveolar and endothelial cells 3) Cytoplasm ofthe
endothelial cell
[edit] Pleura
Serous membrane covering the lung that serves to facilitate sliding during respiration
[edit] GI
[edit] Hard palate
- Parakeratinized = for dealing with rough surfaces
[edit] Tongue
- Filiform papillae provide roughness.
- Filiform are rod like and have a parakeratinized surface.
- Fungiform papillae have bulbous ends.
- These are the red bumps on our tongues.
- Circumvallate papillae
- Valleys are flushed by serous glands called glands of Von Ebner.
- Geographic tongue
- A kind of psoriasis of the tongue.
[edit] Gingiva
- Gingiva = gums of the mouth.
- The epithelial attachment of gottlieb is where the gum epithelial cells attach to the mineral surface of the tooth.
- Enamel covers the crown.
- Mostly appetite
[edit] Periodontal ligament
- Run between cementum and the alveolar bone.
bundles of collagen
- This is a type I collagenous structure.
- Scurvvy: teeth fall out because ligament doesn't get regnerated becuase new collagen can't be generated.
[edit] Gut tube
[edit] Esophagus
- Statified squamous non-ker epithelium (like oral cavity)
- Lamina propria:
- Has no glands (for the most part)
- Muscularis mucosae: has longitudinal muscle, not continuous
- The submucosa does have glands
- Muscularis externus
- Have inner circular and outer longitudinal muscle layers
- Serosa
- Mostly just CT (adventitia)
- Where it penetrates the diagragm (last part), has a mesothelia covering (so a true serosa).
[edit] Stomach
- Txn from esoph to stomach: squamous non-kera epith to simple columnar epithelium.
- Also, we start to see tubular glands.
- Lned with surface mucus cells.
- Surface invaginates into pits where glands empty.
- The cardia has pits with some mucusy glands.
- The fundus / body have glands with a neck and base (base stains more basophilically).
- At the base, staining dark are chief cells that make pepsinogen so they have lots of protein production and packaging stuff that makes them dark.
- Parietal cells of the fundus / body make acids.
- The neck region has parietal and undiffed cells.
- Base has parietal and chief cells.
- The pylorus has deep pits and shorter mucusy glands.
- Pylorus into the small intestine:
- Pits and glands convert into villi.
[edit] GI - Small intestine through anus
[edit] Stomach
- Stem cells:
- Live in the neck of the gland
- Mucus neck cells:
- Usuaqlly in neck
- Can't be distinguished versus stem cells
- Parietal cells = oxynctic cells
- In the neck
- Make acid and intrinsic factor
- Intrinsic factor binds b12 to protect fromn degradation so it can be absorbed later.
- Without factor I you have pernicious anemia (because it is hard to fix)
[edit] Stomach images
- Parietal cells:
- Look like fried eggs.
- Acid secretion:
- Occurs by fusion of tubulovesicles with a secretory canaliculus.
- Bicarb moves in the opposite direction of acid.
[edit] Chief cells
- Produce, store, and then secrete pepsinogen (a zymogen).
- Pairetal cells live in both the neck and the base of the gland.
[edit] Enteroendocrine cells
- ADUP-type (amine precursur uptake and decarobxylation): the name for production of hormone produced by these cells.
- Scattered through epithelium
[edit] Pylorus
- Has deeper pits and shorter glands.
- Has lot sof mucus cell sint eh glands.
- In the pylorus, gastrin is secreted.
- Gland types:
- Cardiac, fundus, body: gastric glands
- Pylorus: deepr pits and mostly just mucus glands
[edit] Small intestine
- Absorptive cells are called enterocytes.
- The muscularis mucosa and lamina propria are folded within the plicae circulares.
- Note that caps in the vili are pretty leaky (fenestrated) but not leaky enough for chylomicrons to get through, hence they gro throug the lymp.
[edit] Glands of the si
- These are simple tubular glands
- Called crypts of lieberkuhn.
- SI:
- crypts are the locaiton of stem cells
- At the very base are paneth cells
- may be important for crohn's disease.
- LI:
- Called Brunner's glands
- Muscularis externa:
- Inner cicrular, outer longitudinal.
- Nerve plexuses:
- Myenteric plexus = auerbach's: between the two layers 9of the musculara externa (inner cirular, outer longitudinal) = intrinsic innervation
- Meissner's plexus = submucosa: runs within the submucosa = extrinsic innervation
- Both are found in the large and small intestine.
[edit] Large intesstine
- Mucosa:
- There are simple columnar absorptive epithelial cells
- Muscularis externa:
- Has outer longitudinal muscle with special bands called tenia coli.
- Haustra are pouches that are formed.
- This is a thickening of outer longitudinal muscle bands.
- the poutches help hold the food material as it is turned into feces.
[edit] Cell turnover
- 3-6 days in SI
- 4-8 days in LI
[edit] Glands
[edit] Salivary glands
- Demilunes
- Here serous cells are found
- Two types of ducts:
- Intercalated
- Striated
- Also there are interlobular ducts in the connective tissue.
- Also called excretory
[edit] Salivary
- Three major are parotid (almost all serous cells), submandibular (mixed serous and mucous) and sublingual (more mucus than serous so will often look just
mucus).
[edit] Saliva
- Low in Na because it helps the taste bud fxn.
- Alkaline, has bicarb to buffer acid
- Protein components:
- Proline rich protiens
- Abundant
- Antibacterial
- Help coat the tooth and keep bacteria off
- Proline rich protiens
[edit] Secretion
- Serous produce fluid, protein, and zymogens
- Mucus produces mucins
- The products mix and then pass through intercalated ducts and then striated duct.
- Striated ducts are most important for removing most Na+.
[edit] Summary
- Most important is to know all glands have intercalated and striated and to know which type of secretion they generate.
[edit] Pancreas
- Two portions: endocrine and exocrine (acinar).
[edit] Endocrine
- Beta = insulin
- Alpha = glucagon
- Delta = somatostatin
- F cells generate pancreatic polypeptide.
[edit] Exocrine pancreas
- Looks like the parotid in that it is made of acini.
- But has not fatty tissue
- Has no striated ducts
- Cholesystokinin = the hormone that moves the gallbladder
- Proteases are the main product of serous cells in the pancreas:
- Trypsin
- Elastases
- Protease E
- Kallikrine
- alpha amylases
- Lipases
- nucleases
- Secretin stimulates ductal cells to generate the volume (water and salt)
- Acinar cells:
- Well staining cyto and open nuc
- These cells have lots of rER and secretory granules.
[edit] Liver
- 70-80% of the blood comes from the hapatic portal vein.
- The rest is oxygenated and comes from the hapatic artery.
[edit] Hepatic blood flow
- Blood spaces are sinusoids, a form of sinusoidal capillary
- Incompletely lined with endothelium; the endothelial cells don't bind to one another
[edit] Formation of bile
- Three ways to understand how hepatocytes filter blood and produce and bile:
- Liver can be lobulated:
- The liver is divided into lobules that have protal veins, hepatic arteries and ducts at one side and
- Portal lobule
- Lobule is defined as all the hepatocytes that contribute bile to a given bile duct
- Hepatic acinus
- Lobule defined by hepatocytes' blood source
- Liver can be lobulated:
[edit] Gallbladder
- Epithlium of gall bladder are simnple columnar epithelium
- Has brush border
- Muscularis:
- True serosis is present
[edit] Bone and Cartilage
[edit] Bone cells
[edit] Osteoclasts
- Rank ligand essential for diff into preosteoclast and full osteoclast.
- Made by osteoblasts
- OPG
- A decoy receptors for rank ligand
- Generates a "focal zone"
- Enzymes for resorption:
- TRAP = Tartrate-resistant alkaline phosphatase
- Can be assessed in blood to know how much activity of osteoclasts is occurring
- Cathepsin K
- TRAP = Tartrate-resistant alkaline phosphatase
- Houshets lacuna
- Well in bone where resorption has occurred.
[edit] Osteoblast
- Come from mesenchymal cells
- Need runks2 and ostrix
- Alkaline phosphatase is key protein involved in matrix production
[edit] Osteocyte
- Connected to each other and to the bone surface by filopodial processes
- These live in channels called canaliculi
- Connected via gap junctions.
- There is one osteocyte per lacunae.
[edit] Genetic profile differs from osteoblasts
- Sclerostin is a gene unique to osteocytes and not osteoblasts.
[edit] Bone matrix
- Made of type 1 collagen.
- A fibrous collagen.
- C-propeptide in the blood means there is bone formation
- Non-collagenous components:
- Know osteopontin, osteonectin, and ostecalcin
- Osteocalcin is a biomarker that can be measured in the blood to know how much osteoblast activity (bone formation) there is.
[edit] Tyeps of bone tissue
[edit] Woven bone
- AKA primary bone and Immature bone
- Not very common in the adult skeleton
- Found only when there is a bone injury or in some pathological state
- This is the type of bone that is formed when you need bone right now.
- Provides rapid stabiliation
- Not very organized
- Osteoid spit out in all directions
- Relatively low mechanical strength.
[edit] Lamellar bone
- AKA secondary bone, mature bone
- Higher mecanical strength than primary.
- Lamellae:
- Differing patterns of collagen organization
- Result in high birefringence
- The ability to refract light differently.
- This is a product of the alternating pattern of collagen fibers.
[edit] Bone anatomy
- Cortical is called compact
- This is the outer part
- Main fxn is structural
- PRovide resistance to loading
- Cancellous bone = spongy bone = trabecular
- NOT squishy
- Small piece of cancellous and small piece of cortical would looke the same.
[edit] Haversion systems
- AKA osteons
- Perferating canals = volkman (?) canals
[edit] Periosteum
- This layer is tightly adherent to the bone
- Both cellular and fibrous layers
[edit] Endosteum
- Endosteum is similar to periosteum but is on the inside of the bone
- Endostium has only cells.
[edit] Structures
[edit] Cartilage
[edit] Hyline cartilage
- Made of type 2 collagen fibrils
- Proteoglycan aggregates
- Chondronectin
- Holds cells in place on the collagen and proteoglycans
- Condrocytes:
- These units are called isogenous groups.
- Hyline matrix:
- Terirtorial matrix = capsular
- Found just around a chondrocyte
- INterteritorial matrix
- Found farther out.
- The collagen fibrils are smaller in the territorial matrix
- Terirtorial matrix = capsular
- Perichondrium
- Fibrous tissue that lines the outside of hyline cartilage in most but not all locations of hyline cartilage
- Allows connection of muscle to cartilage
- Supplies the cells that can differentiate into chondroblasts (which diff into chondrocytes)
[edit] Fibrocartilage
- Found mostly in IV disks
- Has type 1 collagen in it.
- Forms fibers called rows or chords
- Has no perichondrium
[edit] Elastic cartilage
- Found in the ear, epiglottis, and the larynx
- Has type 2 collagen
- Elastic fibers also present
- Has a pericondrium
- Looks much like hyline but you can see fibers in it.
[edit] Joints
[edit] Synarthroses
- Very little movement
[edit] Diarthrovidal (synovial) joints
- Have a cavity with fluid
[edit] Articular cartilage
- This is hyline cartilage
- Articular cartilage has type 2 collagen with fibrils not fibers.
- There are several zones:
- Superficial zone: in intimate contact with the cavity; has very few condrocytes; mostly type 2 collage fibrils; resists sheer forces
- Intermediate (transitional) zone: a transition from superficial to radial
- Radial (deep) zone: large number of chondrocytes; large number of collagen fibrils; compresses to absorb forces; lysogenous groups; interterritorial
regions, etc.
- Calcified zone: interface between unminderalized cartilage of the radial and the subchondrial bone (first part of the zone); distinct from radial by way of
tidemark
[edit] Joint capsule
- Capsule is continuous with the periosteum
- Periostium has two layers: fibrous and cellular layer
- As it starts to cover the capsule, though, it loses it's cellular layer.
- Surface of the synovial membrane is not covered with epithelial cells
- A rare occurance in the body.
- Type A Synoviocyte:
- Found on surface of the synovial membrane
- Look like epithelium but are not because they are not connected together.
- Act like macrophages to detect and phag foreign particles.
- Uusually 1 cell deep but can be 2 to 3 deep.
- Type B Synoviocyte:
- A fibroblast like cell
- Makes hyaluronic acid
- Look much like fibroblasts
- No perichondrium on ends of bones
[edit] Osteoarthritis and Rheumatoid arthritis
- OA:
- Mechanical
- Rubbing of bone without cartilage
- RA:
- INflammation occurs
- osteoclasts "wreak havoc" on the cavity
[edit] Chondrogenesis
- When matrix made "within" existing cartilage, the cells will separate from one another; this is how the bone is lengthened at the epiphyseal plates.
- When matrix is grown around a chondrocyte the cells do not move (adding new matrix onto a surface that already exists); used in lung tissue development.
[edit] Intramembraneous bone formation
- In craniofacial bones, mesenchymal cells get the signal to be osteoblasts, then aggregate to form a bone blastema.
- Then they secrete matrix to form the primary bone tissue with osteoblasts around the outside and some osteobalsts in the middle.
- Bone spicules are formed through intramembraneous ossification.
[edit] Endochondrial bone formation
- Here we start with a template on and in which we build bone.
- There are 5 zones.
- Zone of rest: (shallowest from the articular surface to the middle of the bone): no activity by chondrocytes
- Zone of proliferation: chondrocytes become align and form rows and columns of chondrocytes (stacked coins) which contributes to bone growth as they push
the entire bone unit to increase in length
- Can see rows of fairly flat chondrocytes.
- Zone of hypertrophy: all the chondrocytes hypertrophy; causes bone lengthening
- Has larger cells than proliferative, but still has a similar staining matrix (lightness versus darkness).
- Zone of calcified cartilage: chondrocytes start to die off as they can't get nutrients
- Distinct from the zone of hypertrophy because the matrix is much darker.
- Zone of ossification: bone formation on top of the calcified cartilage template; cartilage is resorbed by osteoclasts.
- Distinct from the zone of calcification because the matrix turns light again (but a little different coloring than the proliferative).
[edit] Fracture healing
- Two types: primary and secondary.
- Primary
- occurs when you have a very stable fracture; no movement (perhaps because of a plate and screws put in by orthopod).
- Then bone can form without fibrous tissue or cartilage formation.
- Can get osteons to remodel through the fractured zone.
- There is no additional tissue being formed (see secondary repair)
- Secondary bone healing:
- Useful when fracture is unstalbe; whats happening when casted
- Bone and cartilage formation occur
- The more stable (the less strain) the less cartilage, the more bone.
[edit] Bone remodeling
[edit] Cartoon
- The reversal zone is where the osteoclasts are not resorbing but the osteoblasts have yet to arrive.
- This process is not limited to trabechular bone but also in cortical bone.
[edit] Activation
- The two major signals are death of an osteocyte or a microcrack in the bone.
[edit] Reversal
- First the osteoblasts must clean up
- They lay down a very thin matrix called the cement line.
- Once they reach near the normal bone surface, the last osteoblasts become lining cells.
[edit] Bone modeling
- This is uncoupled: osteoblastas and clasts don't work in concert.
[edit] Bone remodeling as a cause of bone mass loss
- Estrogen inhibits bone resorption so when estrogen goes away, bone resorption increases.
[edit] Diagnostics
[edit] Pharma
- Anti-resorptives
- Disphophonates
- Cacitonin
- Denosumab
- A monoclonal antibody taht targets RANK-L.
- Recall that RANK-L is required for dev of osteoclasts
- Acts like OPG? (what was the endogenous decoy for RANK-L)?
[edit] Ca, VitD, and Phosphate
[edit] Ca
- Without enough calcium you have rickets (in kids) and osteomalacia (in adults).
- Here the bone doesn't mineralize.
- Deformities occur
- Rickets is reversible if you treat before closure of the growth plates.
[edit] Hypercalcemia
- Primary:
- Elevated PTH
- When using PTH is used as pharam, it is pulsatile--fast rise, fast fall-- it stimulates osteoblasts.
- Treatment
- MOdulate the parathyroid gland
- SEcondary
- Three examples: toxic levels of VitD, immobilization, or malignancy.
- Use antiresorptive pharma.
[edit] Hypocalcemia
- TX:
- Increased Ca and VitD.
[edit] VitD
- Calbindin is required for good CA++ absorption at the gut and VitD is a txn factor modulating txn of Calbindin.
- 1 alpha hydroxylase at the kidney can be deficient to lead to VitD deficiency.
- Ultimatley these cause osteomalacia and rickets.
[edit] Phosphate
- REgulation is athe kidney
- ABsorbed in the duodenum
- Can be CA dependent or independ
- Also regulated by vitD.
- Osteocytes secrete FGF23 and PTH from parathyroid act on kidney to reduce Na-Phosphate co-transporter
- Causes icnreased loss of phosphate.
- When phosphate is low:
- Vitd 1,25 goes up (increase resorption at the gut)
- PTH and FGF23 go down (decrease loss at the kidney)
- When phosphate high:
- vitd 1,25 is low (lower resportion at the gut)
- Increased PHT and fGF23 (increase loss at the kidney)
[edit] Urinary 1

[edit] Cortex and Medulla
- Urine is produced by lobes which contain a single renal papillum which dumps urine into the pelvis which dumps into the ureter.


[edit] More on macrostructure
- Medullary pyramids are separated by renal columns of Bertin.

[edit] Uniferous tubule function

[edit] Uriniferous tubule layout and embryonic development
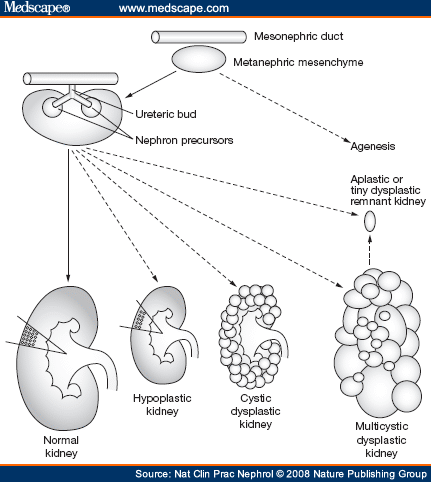
- The first form of a plasma filtering mechanism in the developing human embryo is called the mesonephric kidneys.
- Mesonephric kidneys reach their maximum size at 8 weeks and then undergo a large change and eventually are not kidney-like at all.
- Parts of the mesonephric kidneys persist in men to form:
- the efferent ductules,
- the epididymis,
- the ductus deferens, and
- the ejaculatory duct.
- so, everything after the straight tubules.
- The metanephros gives rise to the permanent kidneys.
- The metanephros contains the metanephric mesenchyme and the uritic bud.
- The uritic bud and the metanephric mesenchyme are both composed of epithelial cells.
- The uritic bud grows up into the nephrogenic mesoderm which is part of the metanephros.
[edit] Uritic bud and nephrogenic mesoderm interaction
- The uritic bud grows into the nephrogenic mesoderm to form the mature uriniferous tubules.
- The interaction between the uritic bud and the nephrogenic mesoderm is called reciprocal induction.
- As the uritic bud grows into the nephrogenic mesenchyme, the uritic bud is the primary epithelial cell tubule structure that will become the
collecting duct.
- Recall that mesenchymal cells are connective tissue cells.
- Recall that mesenchyme looks like loose connective tissue with lots of spindly, undifferentiated cells within.
- Renal corpuscles develop along the length of the uritic bud (that is, the developing collecting duct) and therefore can originate from the tip of the uritic
bud or from epithelium that develops along side the uritic bud.
- Renal corpuscle and nephron development from the tip of the uritic bud:
- At the tops of the uritic bud, mesenchymal cells of the nephrogenic mesenchyme condense and are induced to make a mesenchymal-epithelial
transition (MET).
- Condensation includes proliferation
- These MET cells will become the epithelial cells of the glomerular capsule.
- The bud tip then expands to develop the PCT (proximal convoluted tuble), loop of Henle (LoH), and the DCT (distal convoluted tubule).
- The MET shifted cells of the early glomeruli recruit the formation of blood vessels that will become the glomerular capillaries.
- Renal corpuscle and nephron development adjacent to the uritic bud:
- Along side the uritic bud, epithelial tracts form as S-shaped or comma-shaped tubule structures.
- The tops of these se epithelial tracts will become the glomeruli and the length will become the PCT, LoH, and the DCT.
- The s-shaped buds from condensation, proliferation, and MET of mesenchymal cells will form the PCT, LoH, and DCT.


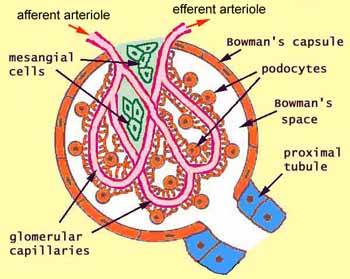
[edit] Renal corpuscle structure
- Note that podocytes are a type of epithelial cell.
- Capillaries are a type of endothelial cell.
- Within the capillaries as they develop within the glomerular tuft, there is connective tissue holding the capillaries in place.
- This connective tissue is called mesangium.
- The visceral bowmans capsule is made up of podocytes.
- Often there is pink material in the bowmans space; it is brush border from the proximal tubule that has washed backward during fixation.
- The distal convoluted tubule (which is, like the PCT, made up of epithelial cells) passes by the afferent arteriole along side the glomerulus.
- The DCT has specialized cells called 'macula densa cells on the surface that is closest to the afferent arteriole.
- Macula densa cells release signals PGE2 to cause the afferent arteriole to vasodilate and ATP to cause the afferent arteriole to constrict.
- Macula densa cells are more columnar, stain darker, and have rounder nuclei than the endothelail cells of the DCT.
- Juxtaglomerular cells (also called granular cells) are endothelial cells of the afferent arteriole that contain granules of renin.
- Granular cells (AKA juxtaglomerular cells) have a large, flattened nucleus, that is more prominent than the nucleus of lacis (extraglomerular mesangial)
cells.
- Granular cells release their renin upon PGE2 binding their EP4 receptor.
- Recall that renin will activate angiotensinogen leading to angiotensin 2 and systemic vasodilation.
- Lacis cells (also called extraglomerular mesangial cells) hold the DCT, the afferent arteriole, and the glomerulus together.
- Extraglomerular mesangial cells may also have some functioning in modifying the signals released by the macula densa cells as they travel to the granular /
endothelial cells of the afferent arteriole.
- Lacis cells (extraglomerular mesangial cells) are found between the macula densa cells and the afferent arteriole endothelial cells.
- Lacis cells have a lighter stain and less prominent nucleus as compared to granular (juxtaglomerular) cells.
- Extraglomerular mesangial cells are found between the convoluted capillaries, too, and serve to hold the loops in their structure.
- In this case, the mesangial cells are located within the basement membrane.
- Lacis cells can send processes into the lumen of the capillaries between the endothelial cells.





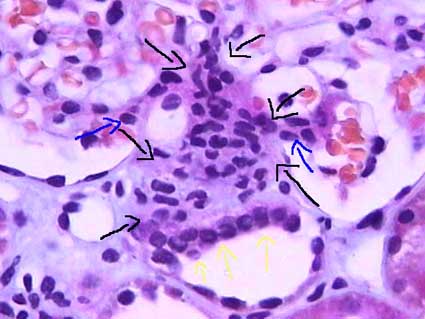
[edit] Forming a filter at the capillary-Bowman-space junction
- The filtrate must first get through the endothelium of the capillary, then through the basement membrane, and then through the feet of podocytes.
- The endothelium of glomerular capillaries is fenestrated without diaphragms to allow only very small proteins and smaller molecules through.
- The basal lamina restricts even the smallest proteins.
- There are three layers to the basal lamina (basement membrane) of the glomerulus.
- The three layers are probably only separate in slides as a result of processing, but they are still effective markers for pathology.
- The lamina rara extrna is farthest from the lumen of the capillary.
- The lamina rara interna is closest to the lumen of the capillary.
- The lamina densa is between the lamina externa and the lamina interna.
- These layers appear as a light-dark-light pattern in EM.
- Podocytes are a type of epithelial cell that provide the finest level of filtration (slit pore diaphragms) of the plasma as it crosses into the Bowman
space.
- Water and small molecules pass freely into the Bowman space.
- It is still disputed what factors play the primary role in keeping proteins from entering the filtrate.
- Some say the anionic charge of the basement membrane, which would repel proteins which are generally negatively charged, is the primary factor that hinders
protein passage.
- Others point to the podocyte processes and the important proteins that make up the processes (ZO1, nephrin, Neph1) as the primary protein-hindering
mechanism.
- Nephrin seems to form a lattice between podocyte processes that would prevent proteins from passing into the bowman space.

- Recall that ZO1 is associated with tight junctions.
[edit] Mesangial cells
- Recall that mesangial cells reside between capillaries within the basement membrane.
- Recall that basement membranes are always made of type 4 collagen!
- Mesangial cells may modulate capillary blood flow.
- Mesangial cells may also act as phagocytes within the basement membrane of the glomerulus.
- Mesangial cells reaches out and cups each capillary around it.
- Mesangial matrix is made up of collagen, glycans, proteoglycans, etc.



[edit] The proximal tubule
- The proximal tubule is characterized by being large, being eosinophilic (cuboidal, continuous, uniform), and having central nuclei.
- The proximal tubule demonstrates cells with brush border and basolateral membrane folding in order to increase its surface area.
- Note that during fixation, the brush border often sloughs off into the lumen.
- The proximal straight tubule continues through the outer stripe of the outer medulla.
- "Straight segments ... terminate at a remarkably uniform level ... that establishes the boundary between the inner and outer stripes of the outer ...
medulla." per wikipedia
- Note that this is true for both cortical- and juxtamedullar glomeruli-derived proximal straight tubules.
[edit] Cell distinction along the PCT, LoH, and DCT
- Recall that the cells of the PCT, LoH, and DCT are all epithelial cells specialized for reabsorption and / or secretion.
- There are four regions that can be distinguished by cell morphology and characteristic: PCT / thick descending limb, thin descending / thin ascending, thick
ascending / DCT, and the collecting duct.
- Note that the thick descending tubule is the same as the proximal straight tubule; the same goes for the distal region: distal straight tubule = thick
ascending tubule.
[edit] Cells of the PCT and PST
- Note that the PST = proximal straight tubule = thick descending / proximal loop.
- There are only epithelial cells in the PCT and thick descending loop.
- Epithelium of the PCT is a simple squamous epithelium.
- the cells of the PCT and thick descending tubule are the only cells with a brush border.
- Cells of the PCT and thick descending tubule also have nuclei that are spaced far apart.
- PCT / thick descending tubule epithelial cells stain very pink.
[edit] Cells of the thin descending and thin ascending tubules
- There are only epithelial cells in the thin descending and ascending tubules.
- Recall that the descending loop is passively, highly permeable to water and solutes.
- Recall that the ascending loop is impermeable to water and actively secretes Na and Cl.
- The epithelial cells of the thin regions are thin cells that stain lightly.
- The nucleus of epithelial cells of the thin tubules is smaller than other nuclei of tubular epithelial cells.
[edit] Cells of the DST and DCT tubules
- The epithelium of the DCT and thick ascending tubule is thicker than the PCT and thick descending tubule.
- cell types in the thick ascending and DCT tubules: epithelial cells, macula densa cells, and principal cells, intercalated cells.
- Epithelial cells of the thick ascending tubule and DCT need lots of protein to facilitate ion transport and so it makes sense that thick ascending
epithelium and DCT epithelium have lots of mitochondria.
- Epithelial cells of the thick ascending tubule and DCT have apical nuclei that bulge outward (perhaps because of the mt that are pushing them
apically).
- Macula densa cells appear at the last part of the thick ascending tubule.
- The DCT is the first site of intercalated cells.
[edit] Differentiating PST and DST
- Thick descending epithelium stain darker than thick ascending epithelium.
- Thick descending epithelium has more basally located nuclei while ascending epithelium have apically located nulcei.
- Thick descending has a thicker wall than the thick ascending.
[edit] PCT versus PST and DCT versus DST identification
- Note that PST and PCT can be differentiated because they are never found in the same location: PCT is in the convoluted area and PST is only in the
medullary ray area.
- Because the DCT and DST are both bound in the cortex, it is likely impossible to tell them apart (unless the structure in question runs right up next to a
glomeruli and has macula densa at which point we know it is a DST).


[edit] Differentiationg PCT and DCT
- PCT and DCT can be distinguished by their stain and size:
- PCT epithelium has a brush border but DCT epithelium does not, though often the brush border is not preserved.
- PCT stains darker than DCT, though sometimes it can be the opposite, so good luck with that.
- PCT is made of larger cells than DCT (so with PCT you travel farther around the tubule before finding the next nucleus).

[edit] Cells of the collecting duct
- Epithelial cells of the collecting duct bulge into the lumen.
- Epithelial cells of the collecting duct have clear distinctions between each cell and have nuclei that do not bulge (like PCT / thick ascending tubule
epithelial cells).
- Nuclei are more basal and irregularly shaped.
- Principal cells are hormonally controlled for water reabsorption and are the major site of potassium regulation.
- Principal cells absorb Na and secrete K.
- Principal cells are generally impermeable to water but can become water absorptive when ADH is present (think AQ2).
- Intercalated cells stain darkly, bulge a little into the lumen, have no brush border, have a more apical nucleus than principal cells, and are the
site of pH regulation.
- There are three sections to the collecting duct: the connecting tubule and cortical collecting tubule, the outer medullary collecting tubule, and the
inner collecting tubule.
- The two proximal sections (connecting duct / cortical collecting duct and the outer medullary collecting duct) have principal and interstitial cells;
the inner medullary collecting duct has only principal cells.
- The inner medullary collecting duct is also called the papillary collecting duct.
- The last section of the inner medullary collecting duct is called the duct of Bellini.
[edit] Distinguishing regions of the kidney
- Note that thin segments of the LoH and DCT / PCT never occur in the same area so they can be used to determine the origin of a section.
- Thin loops of Henle are only found in the medulla.
- Recall that the thick proximal tubule terminates at the outer-inner stripe border of the medulla.
- Convoluted tubules are only found in the medulla.
- Thin loops of Henle are only found in the medulla.
- Distinguishing the medulla:
- The inner medulla has only asc / desc thin tubules and the collecting duct.
- The inner stripe of the outer medulla has asc / desc thin tubules, proximal / distal thick tubules, and the collecting duct.
- The outer stripe of the outer medulla has only thick tubules and collecting duct.
- There are no glomeruli in the medulla!
[edit] Urinary 2
[edit] Loop of Henle
- When the NaCl level is high, we want slow the filtrate flow rate so we have time to reabsorb all that valuable NaCl; therefore, when the NaCl level in the
filtrate is high macula densa cells release ATP to constrict the afferent arteriole and decrease GFR.
- Conversely, very little NaCl in the filtrate at the macula densa means that the filtrate has had lots of time to have its NaCl reabsorbed so we can speed up
GFR. In this case, macula densa cells release prostaglandins that cause renin release (and subsequently vasodilation) at the afferent arteriole.
[edit] More kidney superstructure

- Note that the ascending thick tubule is deeper than the descending thick tubule.
- Arcuate vessels follow the boundary of the cortex and medulla, giving off interlobular vessels that give off afferent arterioles and
receive stellate vessels.
- The thick descending tubule = proximal straight tubule = pars recta.
- We can remove kidney stones through a surgery that pierces the cortex, enters a calyx, and uses a probe to grab / destroy the stone. Percutaneous
nephroscopy.
[edit] Renal vasculature
- The order of renal blood flow: renal artery -> interlobar artery -> arcuate artery -> cortical radial artery (imagine these radiating outward from the
arc; used to be called interlobular arteries) -> afferent arteriole -> glomerular capillaries -> efferent arteriole.
- The return route can start from two locations:
- Superficial and mid-cortical glomerulus: (from efferent arteriole) peritubular capillaries
- superficial peritubular capillaries return via stellate veins -> arcuate vein...
- deeper peritubular capillaries return via cortical radial vein -> arcuate vein...
- Juxtamedullary glomerulus: (from efferent arteriole) descending vasa recta -> ascending vasa recta -> arcuate vein...
- Superficial and mid-cortical glomerulus: (from efferent arteriole) peritubular capillaries
- Then both follow the same path away from their respective nephron: arcuate vein -> interlobar vein -> renal vein.

[edit] Cortex organization
- A renal lobule is a unit of renal tissue with medullary ray at the center with cortical radial vessels bounding it on the outsides.
- So medullary rays are the ascending and descending tubules that will run perpendicular to the capsule of the kidney.
- So, a cortical labyrinth is a collection of renal corpuscles with their associated medullary rays.
[edit] Juxtuloglomerular apparatus and the renin-angiotensin pathway
- Renin is released by granular cells (juxtaglomerrular cells).
- Angiotensin 2 causes systemic vasodilation.
- The apparatus contains the afferent and efferent arterioles, the macula densa, and the extraglomerular cells (lacis cells).
- There are also juxtaglomerular cells which are smooth muscle / endocrine cells.



[edit] Post-kidney urinary ultrastructure
- The calyces, pelvis, ureters, bladder, and uretra all have the same histological structure.
- The only exception is that the walls of the ureters become thicker as they continue.
- The calyces through bladder are transitional epithelium with a lamina propria and smooth muscle.
- Transitional epithelium allows these structures to change volume easily, which is most obviously important in the bladder.
- The lamina propria holds the cells together with connective tissue when changing volume.
- The smooth muscle allows contraction for movement of urine along the tract.
[edit] Transitional epithelium of the bladder
- Uroplakins can fold up like a pleat.
- A protein called uroplakin can be moved to the surface or removed from the surface via vesicular movement in order to increase or decrease surface
area.
- Vesicles that contain uroplakin are called fusiform cytoplasmic vescicles.
- Uroplakin, as with all membrane proteins, is generated via the rER and golgi apparatus.
- Where uroplakin is on the surface, the membrane is thicker; there are thinner areas of membrane that are distict in EM of bladder epithelium.



[edit] Smooth muscle of the bladder
- Smooth muscle of the calyces, pelvis, and ureters are helical in pattern.
- Smooth muscle in the bladder is longitudinal and runs in all directions.
[edit] Endocrine histology
[edit] Describe the structural organization of the endocrine system
- Note that exocrine glands secrete onto an epithelial surface that is usually in the form of a duct whereas endocrine glands secrete into the blood stream.
- Furthermore, both exocrine glands and endocrine glands are usually on the outside of the basement membrane relative to the blood.
- Therefore, exocrine glands do not secrete across the basement membrane.
- However, endocrine glands often must secrete their hormones across the basement membrane.
[edit] Define components of the endocrine system
[edit] Describe the embryonic origin, histological organization, and hormone secretion of the endocrine system
[edit] Origin, organization, and secretion of the hypothalamus
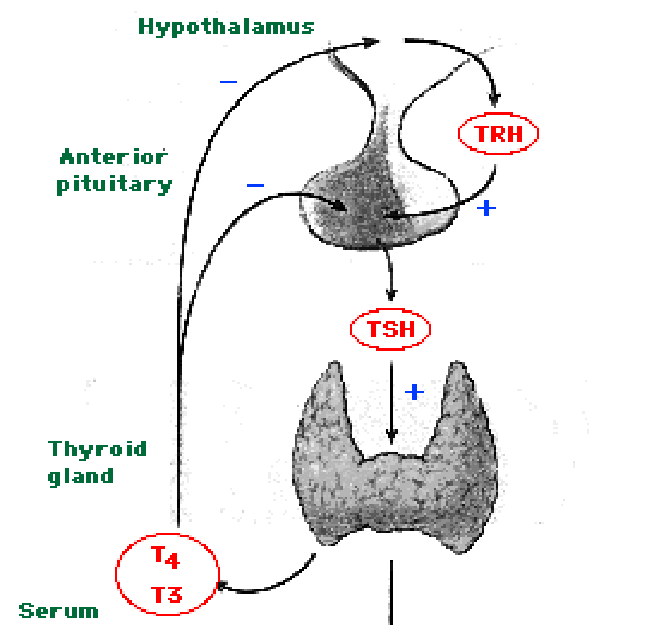
- The hypothalamus releases 5 hormones from three nuclei (dorsal medial, ventral medial, and infundibular nuclei):
- TRH which stimulates thyrotropes and mammotropes (lactotropes) of the anterior pituitary to release TSH and PRL.
- PIF (prolactin inhibitory factor, dopamine) which inhibits lactotropes (mammotropic cells) of the anterior pituitary from releasing PRL.
- SST (somatostatin) which inhibits somatotropes and thyrotopes of the anterior pituitary (adenohypophysis) to release GH and TSH.
- The hypothalamus also contains two more nuclei that produce two other hormones that are delivered directly to the posterior pituitary (neurohypophysis)
through the axons of the neuron cells that produce the hormones.
- The supraoptic neucleus produces vasopressin (ADH, AVP) which acts on the collecting ducts of the kidney (think AQ2).
- The paraventricular nucleus produces oxytocin which acts on the mammary glands (myoepithelial cells) and uterus (smooth muscle cells, contractions).
[edit] Origin, organization, and secretion of the pituitary gland (hypophysis)
- The anterior pituitary consists of the pars distalis, the pars intermedia, and the pars tuberalis.
- The posterior pituitary (neurohypophysis) is made up of the pars nervosa and the median eminence.


- The neuroectoderm (floor of the diencephalon) grows caudally, forms a stalk, and remains attached to the brain tissue of origin.

[edit] Adenohypophysis (anterior pituitary)
- The pars distalis (anterior lobe):
- The pars distalis is composed of fibroblast generated reticular fibers that support hormone-generating epithelial cells and a rich bed of
fenestrated capillaries.
- Cells of the pars distalis can be classified by the way the stain: basophilic, acidophilic, and chromophobes.
- Acidophilic cells: somatotropes and mammotropes (lactotropes).
- Basophilic cells: gonadotropes, croticotropes, and thyrotropes
- Chromophobic cells: stem cells, degranulated cells that would otherwise be chromophilic (see acidophilic and basophilic).
- Differentiating cell types is not possible with light microscope, only by trasmission electron microscopy can these hormone producing cells be
differentiated.

- The pars tuberalis:
- Most cells of the pars tuberalis are basophilic.
- The pars intermeida:
- Colloid-filled cysts fill the pars intermedia.
[edit] Neurohypophysis (posterior pituitary)
- The neurohypophysis contains nerve cells and glial cells (pituicytes).
- The pars nervosa:
- The pars nervosa contains fibroblasts, pituicytes, mast cells and neurons.
- The neurons arise from the paraventricular and supraoptic neuclei where oxytocin and vasopressin are made, respectively.
- These neurons are atypical in that they do not synapse at their distal axons.
- The hormones released by these neurons are stored in granules (called Herring bodies or neurosecretory bodies) at the distal aspect of the axon.
- Herring bodies can be identified under light microscopy.
- The infundibular stalk
- The infundibular stalk, like the pars nervosa, contains atypical nerve axon endings that release hormones.
- The neurons of the infundibular stalk release their hormones into the hypothalamus-pituitary portal system and affect the cells of the anterior pituitary.
[edit] Pituitary portal system
- There are really 4 main components to the portal system: primary and secondary capillary beds, long veins and short veins.
- The primary capillary bed arises from the superior hypophyseal artery and resides around the median eminance.
- The long veins connect the primary capillary bed to the secondary capillary bed.
- The secondary capillary bed resides around the adenohypophysis.
- The inferior hypophyseal artery forms a capillary mesh at the neurohypophysis.
- The short veins connect the capillaries of the neurohypophysis to the secondary capillary bed of the adenohypophysis.
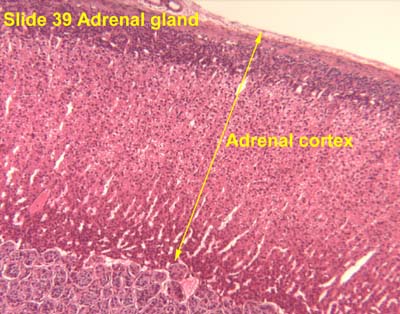
[edit] Origin, organization, and secretion of the Adrenal glands
- The outer shell is made of dense connective tissue that sends septa into the center of the organ as trabechulae.
- Cortex from mesoderm, medulla from neuro ectoderm.
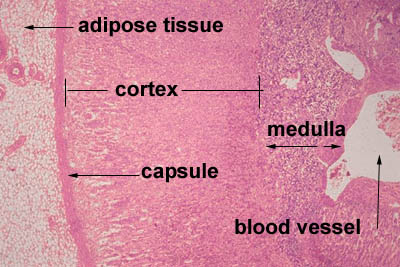
[edit] Adrenal cortex
- GomiFacoRea: glomerulus-mineralocorticoids, fasciculata-corticoids, reticularis-androgens.
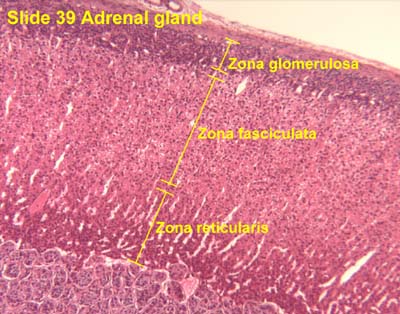
- The glomerulosa:
- The glomerulosa layer is characterized by closely-packed, arched chords of columnar or pyramidal cells surrounded by capillaries.
- The glomerulus can be differentiated from the capsule because of increased cellularity, prominent, circular nuclei, prominent arches, less connective
tissue (which usually stains bright pink).
- The fasciculata:
- The fasciculata is characterized by long chords of polyhedral cellls and fenestrated capillaries.
- The fasciculata can be differentiated from the glomerulosa by distinct change in from short, bulbous cellular collections to long chord-like cellular
collections.
- The reticularis:
- The reticularis can be differentiated from the fasciculata by less organized cellular collections, more eosinophilic staining (pinker, think about the
granules of norepi and epi),



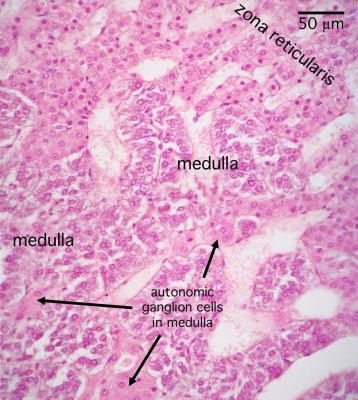
[edit] Adrenal medulla
- The medulla of the adrenal is composed of chromaffin cells which can be considered like post-ganglionic neurons.
- Chromaffin cells can either be norepinephrine producing or epinephrine producing and will have granules full of their labors.
- Norepinephrine-producing Chromaffin cells are found near medullary arteries.
- Epinephrine-producing Chromaffin are found near cortical sinuses.
- Cell density within the medulla is less than that of the cortex.



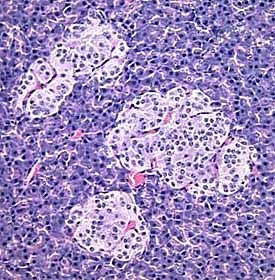
[edit] Origin, organization, and secretion of the Pancreas
- The endocrine portion of the pancreas arises from endodermal tissue near the bile duct.
- The notes also say that the endocrine protion arises from epithelium of the gut.
- There are four cell types in the endocrine islets of langerhans: beta, alpha, delta, and F / pp cells (by abundance).
- Delta cells make somatostatin.



[edit] Origin, organization, and secretion of the Thyroid
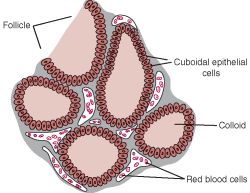
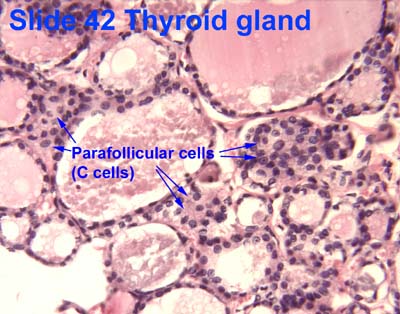
- Within or between the follicles can be found C cells (parafollicular cells) which produce calcitonin.
- Production, storage, and release of thyroid hormones involves both endocrine and exocrine functions.
- Thyroglobulin made in rER, glycocylated in rER / golgi, and moved into the lumen.

[edit] Origin, organization, and secretion of the Parathyroid glands
- The Parathyroid gland arises from the pharyngeal pouches.
- Like the adrenal glands, the parathyroid glands have a capsule with septa that run inward.
- The parathyroid gland is composed of two cell populations: chief cells and oxyphil cells.
- Chief cells:
- Chief cells contain eosinophilic granules of PTH.
- Note that regulation of chief cell PTH release is an inhibition of inhibitoin mechanism: when serum Ca levels decrease, fewer Ca-receptors bind Ca (the
ligand) causing a decrease in intracellular signaling and an increase of PTH release.
- Oxyphil cells:
- The function of oxyphil cells is unknown; however, it is known that they arise during puberty.
- Oxyphil cells are larger than chief cells with an acidophilic cytoplasm and abnormally shaped mt.
- Oxyphil cells are often found in clusters at the center of the parathyroid gland or near the perimeter.




[edit] Primary hyperparathyroidism
- Primary hyperparathyroidism is a defect with the parathyroid itself causing an an elevation of PTH.
- Giving PTH intermittently to post-menopausal women is associated with decreased risk of bone fracture.
- continuous administration of PTH causes bone loss yet intermittent PTH administration causes increases in bone mass.
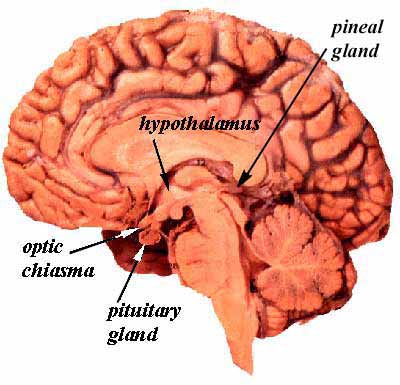
[edit] Origin, organization, and secretion of the Pineal gland
- The pineal gland arises from neuroectoderm from the floor of the diencephalon (just like the neurohypophysis).
- The pineal gland is pine-cone shaped and covered with connective tissue.
- This pine-cone shaped pineal gland is located in the posterior aspect of the third ventricle.
- The pineal gland contains pinealocytes, interstitial glial cells (like astrocytes).
- The pinealocytes produce melatonin and thus take part in daily rhythmicity.
- Rene Descarts explained human behavior and thought via the pineal gland because of its involvement in sensation, imagination, memory, and bodily
movement.
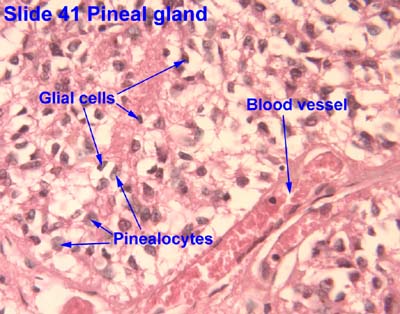

[edit] Origin, organization, and secretion of the Diffuse Neuro-endocrine system
- Organs that have diffuse endocrine tissue include the heart, kidney, thymus, gut, and gonads.
[edit] Bone as an endcrine organ
- Two major signals are released by bone to affect physiology: FGF23 and uOCN.
- FGF23 is released by the bone and causes:
- Kidneys decrease phosphate (Pi) reabsorption resulting in decreased serium Pi.
- Kidneys decrease 1,25VitD activation resulting in decreased serum 1,25OH VitD and decreased Ca reabsorption.
- So FGF23 is generally an anti-bone-building signal.
- uOCN is released by the bone and causes:
- Pancreatic beta cells to increase insulin release resulting in decreased serum glucose.
- Adipocytes to increase adiponectin resulting in changes to glucose and fatty acid catabolism.
- Muscle to increase sensitivity to and uptake of glucose resulting in decreased serum glucose.
- So uOCN is generally a pro-growth-use-up-the-glucose signal.
- Bone also releases osteocalcin which has been shown to be associated with poor fertility when deficient.
[edit] Female reproductive histology
[edit] Ovarian cycle
[edit] Origin and fate of ovarian follicles
- Ovarian follicles are composed of a germ cell (oocyte) surrounded by supporting cells (follicular epithelial cells).
- Primordial germ cells originate from the yolk-sac (endoderm) and migrate to the genital ridge where the ovaries are developing.
[edit] Ovarian anatomy
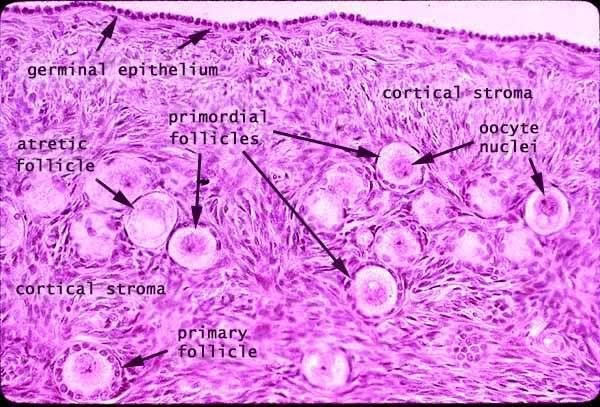
- The cortex epithelium of the ovary is simple cuboidal epithelium.


[edit] Ovarian follicle
- During the ovarian follicular phase mesenchymal cells will differentiate into theca cells to surround the follicle with an extra two layers.
- The endocrine layer of theca cells is called the theca interna; the vascular layer of theca cells is called the theca externa.
- The ovarian follicle has a specific anatomy of layers:
- Within the follicular cell population one may find a Call-Exner body which are collections of granulosa cell membrane with granulosa secretions
within.
- The follicular cells are surrounded by a basement membrane (basal lamina).
- The basal lamina is surrounded by theca cells (from mesenchyme) which form two layers: theca interna and theca externa.




[edit] Stages of the ovarian follicle
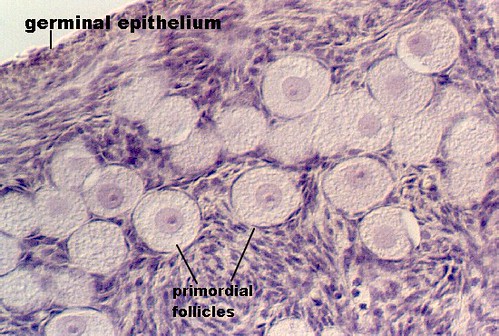
- Developing follicle:
- In the developing follicle, the follicular cells are cuboidal and have proliferated and differentiated into granulosa cells.
- Secondary follicle:
- The secondary (vesicular, antral) follicle is uniquely defined by a developing antrum and a theca externa.
- Granulosa cells secrete stroma-weakening factors to allow expansion of the follicle.
- A primary stroma-weakening factor is plasminogen-activator which converts plasminogen to plasmin (fibrinolysin, a trypsin-like enzyme) which
cuts up fibrin.
- Granulosa cells secrete a meiosis-regulationg factors to inhibit movement from prophase 1 to metaphase 2 in the oocyte.
- It is in the secondary follicle stage (antral stage, vesicular stage) that the oocyte reaches its mature size.

- Mature follicle:
- The corona radiata is sometimes called the rim.
- The cumulus oophorus is sometimes called the stalk.
- Mature follicles are very large: can be over 1 cm!
- As a mature follicle, the oocyte progresses from prophase of meiosis 1 to metaphase of meiosis 2 and thus generates the first polar body.
- Note that having entered meiosis 2, the oocyte is called a secondary oocyte.

http://t0.gstatic.com/images?q=tbn:ANd9GcR_5ajvKIDofXIvPSq_8CEqUgDbjlsBRtYNqKEDaGYemdfQoqPAtQ

- Deciding on which type of follicle you're observing:
- flattened follicular cells: primordial
- cuboidal folliclar cells (with a layer on either side--zona pellucida or basal lamina): developing
- antrum / fluid: secondary follicle
- no way to distinguish secondary from mature.

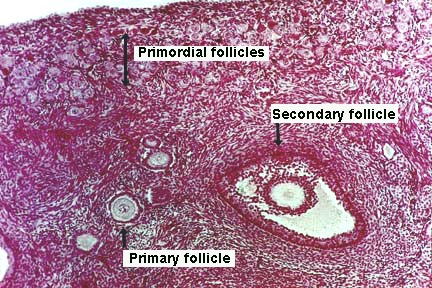
- Here is a good image (though it does not show a mature follicle):



[edit] Endocrine regulation of follicle maturation
- Increased estrogen inhibits FSH release at the pituitary thus stopping the growth of the follicle and allowing ovulation.
- Increased estrogen stimulates LH release at the pituitary thus commencing ovulation.
- Recall these classic images:

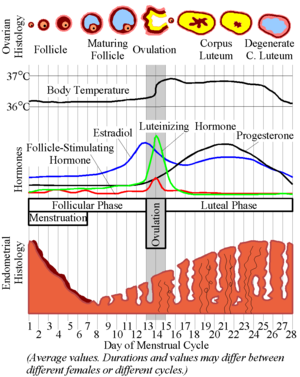
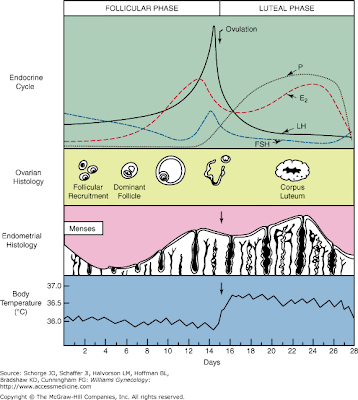
[edit] Ovulation
- As the granulosa cells of the secondary and mature follicle produce more and more estrogen, more and more LH is released from the anterior pituitary gland
(adenohypophysis, pars distalis).
- Ovulation events include:
- Breakdown of the cumulus oophorus, thus the oocyte floats freely in the antrum and follicular fluid.
- Weakening of the ovarian stroma:
- Proteolytic enzymes like collagenase disrupt the stromal connective tissue.
- Granulosa cell connections weaken
- Local ischemia causes a pale spot on the surface of the ovary called a stigma.
- Follicular wall ruptures releaseing an oocyte with the corona radiata and zona pellucida surrounding.
[edit] The corpus luteum
- LH causes granulosa cells to become granulosa lutein cells and theca cells to become theca leutein cells.
- Granulosa lutein cells develop the morphology of a secretory cell and actively produce progesterone.
- Note that the production of progesterone by the granulosa lutein cells is necessary for implantation of the embryo.
- LH causes theca cells to become theca lutein cells.
- If pregnancy does not occur, the corpus luteum is called the corpus luteum of menstruation.
- Therefore, progesterone from the corpus luteum is self limiting.
- That is, the corpus luteum will bring about self-demise via progesterioen inhibition of pituitary-LH unless chorionic gonadotropin is generated by the
placenta.
- If pregnancy occurs, the corpus luteum is called the corpus luteum of pregnancy.
- Recall that trophoblasts of the placenta produce chorionic gonadotropin which maintains the corpus luteum (even though LH drops because of high progesterone
levels).
- hCG is the hormone used to test for pregnancy.
- Granulosa cells of the corpus luteum of pregnancy produce relaxin which has a smooth-muscle relaxing effect (histo says "during parturition",
wikipedia says "during gestation").
- Relaxin opposes the pro-parturition actions of oxytocin; that is, it keeps the smooth muscle of the uterus relaxed.
- Relaxin targets the fibrocartilage of the pubic symphysis to increase articulation.
- Note that physio notes say that the role of relaxin in pregnancy is unclear.

[edit] Follicular atresia
- Follicular atresia generates a long-lasting, scar-tissue structure called the corpus albicans.
- The zona pellucida remains (bright pink) and a wavy line (the basement membrane, called the glassy membrane).

[edit] The uterine tubes
- The uterine tubes are muscular tubes that extend from the ovary on the posterolateral wall of the abdomen to the medioventral aspect of the abdomen and the
lateral aspect of the uterus.
- As the oviducts progresses distally, there are fewer involdings.


http://www.jci.org/articles/view/29424/files/JCI0629424.f1/medium
[edit] Layers of the oviduct
- Like other epithelial tracts there are four major layers to the oviduct (from inner to outer): mucosa, lamina propria, muscularis, and serosa.
- Secretions from the oviduct promote sperm activation.
Does this refer to capacitation?
- Mucosa of the oviduct:
- The mucosa of the oviduct is comprised of columnar, ciliated epithelial cells.
- These columnar cells are secretory and are called Peg cells.
- Estrogen (from the corpus luteum) increases the height of the columnar cells.
- Progesterone (from the corpus luteum) increases the ciliary action of the columnar epithelial cells of the mucosa.




- Lamina propria:
- The lamina propria of the oviduct is highly vascularized.

- Muscularis:
- As with so many muscularis layers, there is an inner circular and outer longitudinal layer.
- The two layers of the muscularis are interwoven.

- Serosa:
- The serosa is a true serosa because it is lined with mesothelium.



[edit] The uterus
[edit] Layers of the uterine wall
- Like the oviducts and other epithelial tracts, there are four tissue-type layers to the uterus which make up three functional layers of the
uterus.
- The epimetrium is composed of serosa and adventitia and is a form of mesothelium as one would expect to cover surface of organs that faces the inside
of the abdomenal cavity.
- Note that the cervix is histologically distinct from the rest of the uterus; we will revisit this.
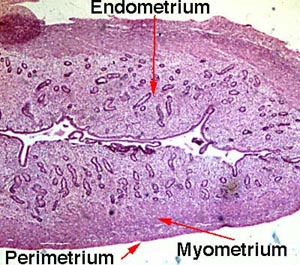



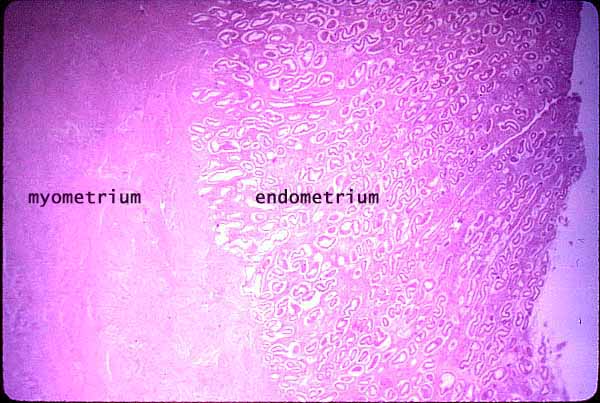


[edit] The uterine cycle
- From physio notes:
- The proliferative stage is characterized by endometrium hypertrophy and formation of spiral arteries.
- So as the ovary is maturing its follicle, the uterus is regenerating it's surface (where the egg will implant) and increasing vascular access to the
surface.
- The secretory stage is characterized by coiling of glands, secretion of mucus, tortuous arteries, and peak thickness of the
endometrium.
- So, as the ovary has shed an ovum and is now increasing hormone production via the corpus luteum, the uterus is using glands and arteries of the uterus to
modify the uterine microenvironment to the optimal conditions for egg implantation.
- The ischemic stage is characterized by arterial constriction, decreased blood flow, and increased prostaglandins.
- So as the ovary has reached its lowest levels of hormone production, the uterus is decreasing nutrition to the endometrium and allowing the mucosa to
undergo necrosis by ischemia.
- The menstrual stage is characterized by desquamation of the endometrium.
- So as the ovary has reached its lowest levels of hormone production, the uterus is shedding its endometrium.
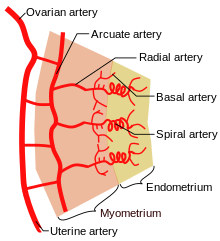
[edit] Uterine vasculature
- The uterus is supplied by arcuate arteries that run along the myometrium layer and by radial arteries that cross into the endometrium.
- The radial arteries give off straight (basal) arteries that supply the endometrium basalis.
- Note that the endometrium is divided into two layers: the endometrium basalis is a constant, mostly unchanging layer while the endometrium
functionalis cycles through generation (proliferation) and shedding.
- There is no structural marker to distinguish between the basalis and the functionalis of the endometrium.
- Spiral (coiled) arteries are heavily muscular, generated during the endometrial cycle, and bridge the radial arteries into the endometrial
functionalis.
- Of special note are the vascular structures nearest the lumen of the uterus called lacunae.
[edit] Uterine endometrium
- The endometrial mucosa contains uterine glands.
- Uterine glands are tubular with many branches.
- Uterine glands contain both ciliated and non-ciliated cells.

[edit] Histological changes in the uterine cycle
- The phases are divided over approximately 28 days: menstruation (days 1-5), proliferation (6-15), secretion (16-17), ischmia (18-28).
- Menstruation:
- Note that the base of the uterine glands remain visible in the endometrium basalis.

- Proliferative stage:
- The proliferative stage is driven by estrogen produced by the developing follicle.
- In addition to gland coiling, spiral arteries develop in the thickening endometrium.
- Cells of the proliferative endometrium accumulate glycogen.
- Secretory stage:
- The secretory stage is driven by progesterone from the corpus luteum.
- The secretory stage is characterized by release of glycoprotein-rich products, swelling and torture of the glands and spiral arteries, and
accumulation of fluid in the stroma of the endometrium.
- Ischemia:
- The ischemic stage is characterized by constriction of the coiled arteries, stromal fluid loss, and lymphocyte / macrophage cell
invasion.
- When the corpus luteum degenerates and progesterone levels drop, local prostaglandins are released into the endometrium, the vessels constrict, and blood
flow is arrested causing ischemia.
- The coiled arteries dilate and constrict intermittently which causes ischemia, cell lysis, a weakened stroma, bursting vessles, and debridement of the
functionalis.
- The arteries both restrict oxygen (constriction) to cause cell death but also to flush away the dead tissue (dilation).
[edit] Uterine cervix
- As mentioned before, the cervix is histologically distinct from the rest of the uterus.
- The cervical myometrium:
- The cervical myometrium has less smooth muscle and abundant collagenous connective tissue with elastic fibers.
- The cervical endometrium:
- The cervical endometrium has denser stroma, simple columnar epithelium, branched, dilated, cyst-forming glands, and longitudinal mucosal
folds called plicae (plicae palmatae).
- Cervical glands can form cysts called Nabothian cysts.
- The cervical mucus:
- Mid way through the cycle (think ovulation and sperm-friend environment) the mucus is watery, contain lysozyme (bacterioalcidal), and promotes
sperm motility.
- This sperm-friendly mucus is estrogen-stimulated.
- Late in the uterine cycle (think corpus luteum and potential implantation) the mucus is viscous and progesterone-stimulated.
- During pregnancy the mucus is particularly thick (think lots of progesterone) and thus protective of the fetus.
- One may look for the loss of this dense mucus plug as a sign that parturition is commencing.
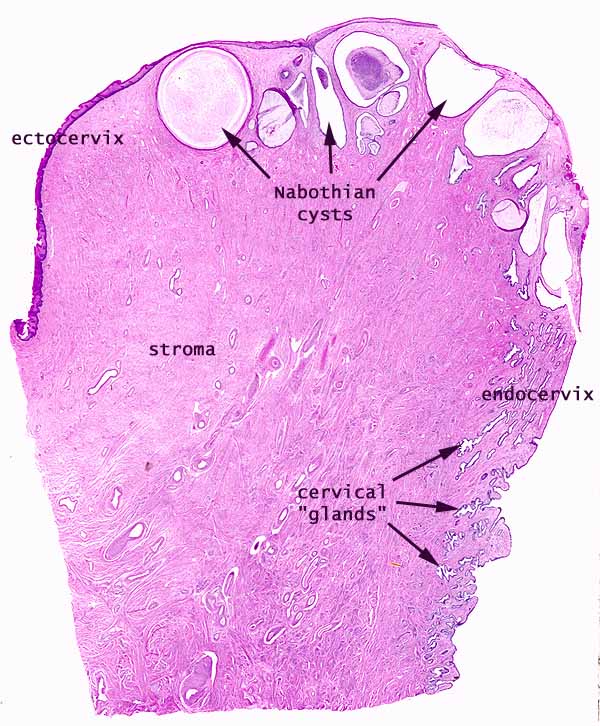
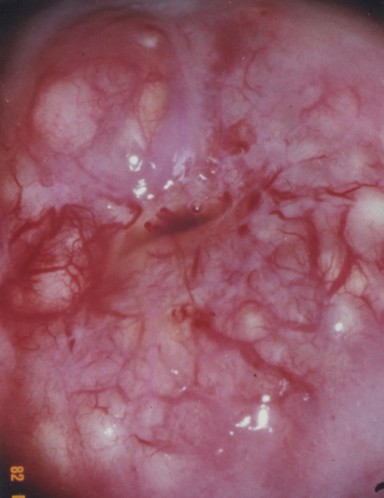


[edit] The ectocervix
- The ectocervix is also called the portio vaginalis.
- At the ectocervix the epithelium changes from columnar (cervix) to stratified squamous (vagina) abruptly.
- Normal ecotcervix:


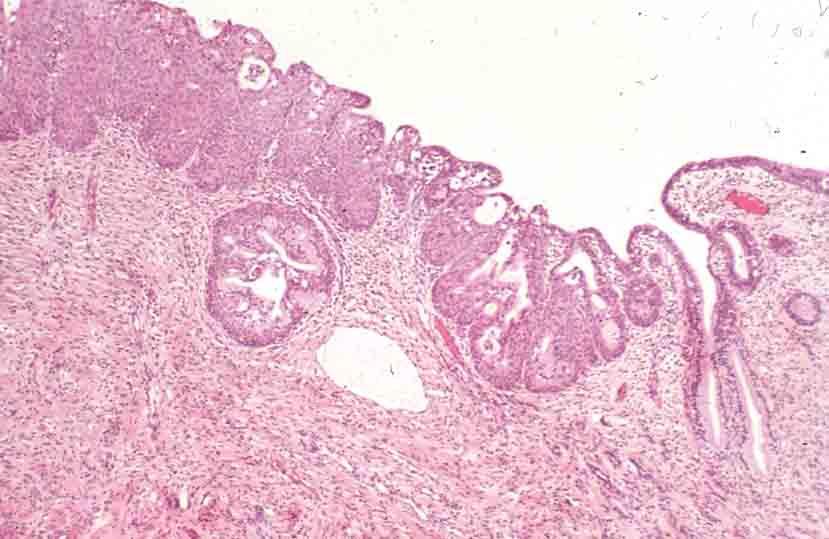
[edit] Vagina
- The mucosa is characterized by stratified, squamous, non-keratinized epithelium.
- The epithelial cells of the vagina--like those of the uterus--accumulate glycogen upon estrogen signaling.
- The vaginal lamina propria has no glands, patches of lymphocytes, and can have folds.
- Recall, however, that the uterus does have glands in the lamina propria.
- The vaginal muscularis has interlacing bundles of smooth muscle.

.jpg)


[edit] Mammary glands
- The mammary glands are made of a compound tubuloalveolar system; that is, there are alveoli that take part in the secretory component and there are ducts
that take part in the transport component.
- A group of about 20 glands forms a mammary lobule.
- Secretory component:
- Milk is generated by cuboidal epithelial cells arranged in alveoli.
- The myoepithelial cells arise from the cuboidal epithelial cells.
- Plasma cells are found in and around the alveoli in order to generate IgA.
- Mammary ducts:
- The epithelium that lines the ductule system is stratified cuboidal.
- There is a significant amount of smooth muscle between the ducts and sinuses.
- Most breast cancers arise from lactiferous duct cells.






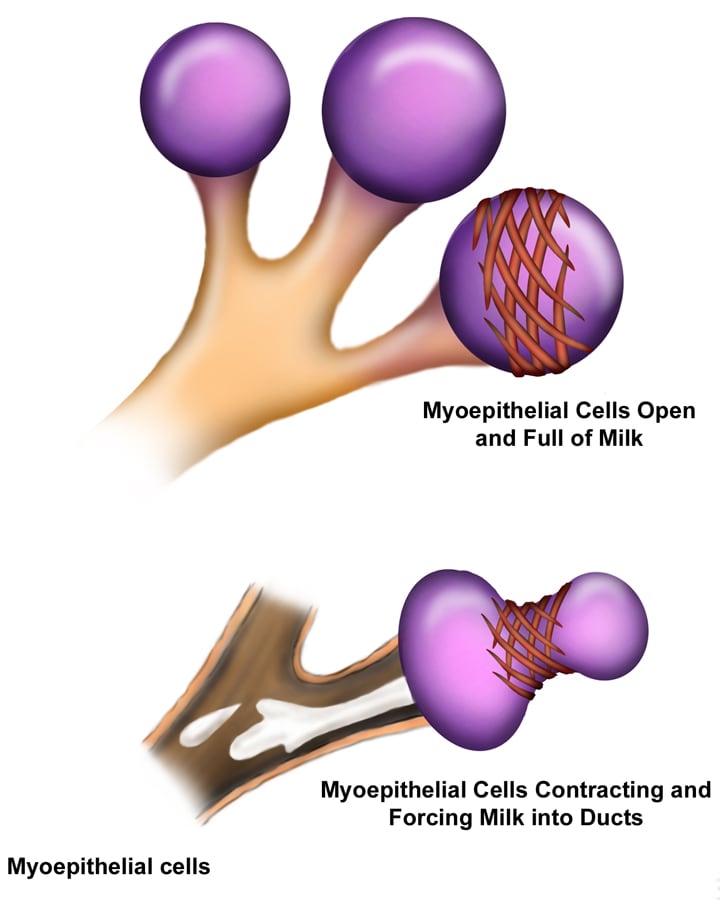
[edit] Mammary glands at puberty
- When estrogen increases at puberty (and prolactin is present), alveolar buds develop and regress with each ovarian cycle.
[edit] Mammary glands in pregnancy
- Upon pregnancy, estrogen is found at high levels along with prolactin, placental lactogen, and progesterone and therefore the alveolar ducts and
alveoli fully develop.
- Lactogenesis is regulated by estrogen, progesterone, and prolactin.
- This makes sense because estrogen and progesterone increase throughout pregnancy and once progesterone and estrogen drop (at parturition), prolactin has
its most potent effect.
- Galactogenesis is maintained by prolactin and oxytocin.

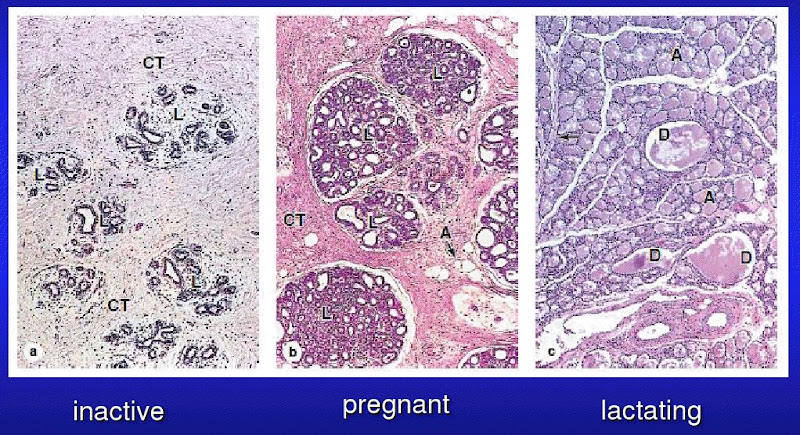
[edit] Breast milk
- The first milk generated is called colostrum; colostrum is lactoprotein- and immunoglobulin- rich and lipid-deficient.
- Lactation can generate 1100 to 2100 ml every day.
- Note that physio said 800-1200 ml / day.
- These cuboidal epithelial cells use several secretion mechanisms to release their products.
- Merocrine secretion is used to secrete casein, alpha-lactalbumin, and PTH-RP (protein, basically).
- Apocrine secretion is used to secret TAGs and cholesterol.
- Exocytosis is used to secrete lactose.
- Transcytosis (from adjacent plasma cells) is used to secrete dimeric IgA.
[edit] Regulation of milk let-down
- Note that afferent CNS signals also stimulate dopamine inhibition such that prolactin is increased which stimulates the cuboidal epithelial
cells of the alveoli to increase milk production.
- Note that multiple neuroendocrine factors have been found besides oxytocin to relax the smooth muscle sphincter between the lactiferous ducts
and the lactiferous sinuses.
[edit] Summary
- Estrogens increase as the follicle develops and:
- inhibit FSH at the pituitary
- stimulate LH at the pituitary
- LH promotes corpus luteum formation
- Corpus luteum produces progesterone and estrogen
- Estrogen causes uterine proliferation phase
- Progesterone causes uterine secretory phase
- Progesterone inhibits LH
[edit] Male reproductive
[edit] Anatomy review
- The mediastinum testis is where the vessels (blood and lymphatics), nerves, and efferent duct enter and exit the testis.
- Note that the mediastinum testis is connective tissue while rete testis is a collecting tubule tissue.
- The tunica propria is the outer wall of the seminiferous tubule and is made of smooth muscle and fibroblasts.
[edit] Seminiferous epithelium

[edit] Spermatogenesis
- Primary spermatocytes are in the prophase of meiosis 1 and stick around for 20 days.
- Secondary spermatocytes are relatively short-lived.
- Cells of the basal compartment: type A and type B spermatogonia, primary spermatocytes
- Cells of the adlumenal compartment: secondary spermatocytes, spermatids, spermatozoa
- Cells and processes: type A spermatogonia undergo mitosis to become ... type B spermatogonia undergo mitosis (and differentiation) to become ... primary
spermatocytes undergo meiosis 1 ... secondary spermatocytes undergo meiosis 2 ... spermatids undergo morphologic modification (differentiation) ...
spermatozoa.
- Spermatocytogenesis includes all the steps that generate an increasing number of cells (that is, type A spermatogonia through generation of secondary
spermatocytes); this makes sense because of the name "cyto" = cell and genesis = "origin of".
- Spermiogenesis is the converse of spermatocytogenesis: spermeiogenesis is the maturation of existing cells into spermatozoa (from the secondary
spermatocyte stage to the spermatozoa stage).



[edit] Mitosis and Meiosis
[edit] Spermiogenesis
- Spermiogenesis is characterized by morphological changes to the spermatid, that is the specialization / differentiation of the spermatid into the
spermatozoa:
- Golgi phase:
- The enzymes like hyaluronidase and trypsin-like protease accumulate at one pole of the nucleus in a vesicle (which will become the acrosome).
- Acrosomal phase:
- The cell rotates such that the axoneme faces the lumen.
- Maturation phase:
- The maturation phase is characterized by motile apparatus development and the capacity to fertilize.
- Note that during the maturation phase, the spermatids are not yet motile or fertile.

[edit] Sertoli cells
- Sertoli cells are characterized by being tall, columnar epithelial cells with a large, indented euchromatic nucleus, and lots of eosinophilic
cytoplasm.
- Neighboring Sertoli cells within a region have gap junctions which suggest that Sertoli cells are coordinated within their region.
[edit] Blood testis barrier
- Sertoli cells of the seminiferous epithelium form tight junctions between one another to keep immunoglobulins in the blood from entering the lumen of the
tubule.
- Note that these tight junctions of the Sertoli cells are on the lumenal side of the spermatogonia.
- These tight junctions define the two compartments: basal compartment and adlumenal compartment.
[edit] Sertoli function stimulated by FSH
- Recall that the anterior pituitary releases FSH which binds to the FSH receptor on Sertoli cells.
- FSH signaling on Sertoli cells causes phagocytic activity and production of several secretions.
- Secretions of the Sertoli cells:
- Activin and Inhibin: stimulate and inhibit the anterior pituitary cells to release FSH.
- Androgen binding protein (ABP): secreted into the lumen of the seminiferous tubule, binds up and concentrates testosterone.
- Tubular fluid: lubrication.
[edit] Leydig cells
- Note that Leydig cells do not secrete activin or inhibin.
- Leydig cells are found in clusters in the peritubular interstitium of the testis, between the seminiferous tubules.
- Recall that Leydig cells are often found near capillaries.
- Leydig cells are characterized by eiosinophilicism, lots of sER, mt with tubular cristae, and a lack of secretory vesicles.
- Regarding lots of sER, recall that steroids are generated in sER.
- Regarding a lack of secretory vesicles recall that steroids can pass directly through the membrane and therefore need not vesicular secretion.
- By transmission EM, one can also discern crystalline inclusions.
- Leydig cells are "transiently" active during development and then initiate full activity at puberty.
[edit] Segments of the male reproductive tract
- Seminiferous tubules -> tubuli recti (straight tubules) -> rete testis -> efferent ductules -> epididymal ducts -> ductus deferens (vas deferens) ->
ejaculatory duct -> prostatic uretral -> membraneous urethra -> penile urethra.
Where do basal cells and principal cells start?
- Straight tubule (tubuli recti)
- Contributes to fluid
- Cuboidal to columnar
- Contains Sertoli cells
- Rete testis
- Contributues to fluid
- Has an irregular epithelial morphology: squamous, cuboidal, columnar.
- Has a fibrous stroma
- Efferent ductule
- Like the rete testis, the efferent ductule has an irregular epithelial morphology and also has ciliated, pseudostratified cells.
- These ciliated pseudostratified cells are called principal cells and are found as clusters of columnar cells surrounded by short cells.
- These cells are the only ciliated cells of the male genital tract; it even makes a bit of sense that these are ciliated because they are passing
- Like the rete testis, the efferent ductule has an irregular epithelial morphology and also has ciliated, pseudostratified cells.
through a sturdy connective tissue structure.
- Ductus epididymis
- The ductus epididymis is characterized by an pseudostratifed columnar epithelium, stereocilia, and a prominent muscularis layer.
- Basal cells are regenerative.
- Principal cells are secretory / absorptive.
- The ductus epididymis has a prominent muscularis layer that thickens distally and changes from just a circular layer to an inner circular and outer
longitudinal layer.
- The functions of the ductus epididymis: absorb 90% of the tubular fluid, secrete factors that mature and capacitate sperm, and phagocytize cellular debris.
- Ductus deferens (vas deferens)
- The ductus deferens is characterized by a pseudostratified columnar epithelium, abundant sympathetic innervation, and three layers to the
muscularis.
- The ductus deferens is pseudostratified (like the efferent ductule and ductus epididymis) and has stereocilia (like the ductus epididymis).
- The muscularis has now added an inner longitudinal muscle layer to the ductus epididymis's inner circular and outer longitudinal.
- The sympathetic innervation will be important for ejaculation which is facilitated by contraction of the ductus deferens's muscularis.
- Recall that Point and Shooting require Parasympathetic and Sympathetic innervation.
- Ejaculatory duct
- Membranous urethra
- The membranous urethra is characterized by a pseudostratified epithelium that transitions to a stratified columnar epithelium.
- Penile urethra
- The penile urethra is characterized by a pseudostratified epithelium that transitions to a stratified columnar epithelium, perhaps continuing on to
stratified squamous epithelium at the top.
- The penile urethra is also characterized by the presence of urethral glands which are clusters of mucus cells in the mucosa.
| Section | Epithelium | Appendages | Muscularis |
|---|---|---|---|
| Seminiferous tubule | stratified | none | none |
| Tubuli recti | cuboidal -> columnar | none | none |
| Rete testis | irregular: squamous, cuboidal, columnar | none | none |
| Efferent ductule | pseudostratified | cilia | none |
| Ductus epididymis | pseudostratified | sterocilia | circular -> circular (inner) + longitudinal |
| Ductus deferens | pseudostratified columnar | stereocilia | longit (inner) + circular + longit |
| Ejaculatory duct | pseudostratified columnar | ? | ? |
| Prostatic urethra | pseudostratified -> simple columnar | ||
| Membranous urethra | pseudostratified -> stratified columnar | none | longit (inner) + circular + longit |
| Penile urethra | pseudostratified -> stratified columnar -> stratified squamous | none | longit (inner) + circular + longit |
[edit] Accessory glands of the male reproductive tract
[edit] Seminal vesicles
- Seminal secretions are rich in fructose (for energy), prostaglandins, amino acids, and ascorbic acid.
- The seminal vesicle has plenty of smooth muscle with which to force this secretion out into the ejaculatory duct.
- Seminal vesicle function and growth is mediated by testosterone.
- The vesicles are blind-ended pouches near the prostate and ductus deferens.
- Seminal vesicles are lined with pseudostratified columnar epithelium.
- This makes sense because they pump semen out into the GU tract at the ductus deferens and ejaculatory duct, both of which are pseudostratified.
- The seminal vesicles have mucosal arches which are highly folded, convoluted walls.
[edit] Prostate
- The prostate generates a secretion that becomes part of the semen and serves to condition the environment of the famale GU tract.
- The fribromuscular stroma surrounding the prostate is important for proper discharge of prostatic secretions during ejaculation.
- Zinc inhibits macrophage activity.
- Fibrinolysin inhibits clot formation in the uterus.
- The prostate as a gland has alveoli connected via tubules; the tubules converge to generate a group of ducts that dump into the urethral crest.
- The urethral crest receives the ejaculatory ducts and becomes the prostatic urethra.
- The epithelium within the gland is irregular: pseudostratified to simple columnar.
- Corpora amylacea is a concentration of glycoprotein-rich secretion in the lumen of the gland that stains eosinophilic (because of the "protein-rich"
part).
- The prostate has three concentric-like zones: central zone, transitional zone, and peripheral zone.
- The central zone of the prostate is the most medial and contains primarily periurethral mucosal glands.
- The central zone often stains the lightest of the zones.
- The transitional zone of the prostate is the middle zone and contains periurethral submucosal glands.
- Note that the central and transitional zones are most commonly associated with benign prostatic hypertrophy (BPH).
- The peripheral zone of the prostate is the outer zone, contains the main glands, is called the prostate proper, and is commonly involved
with malignancies.
- The peripheral zone is often involved with prostate cancer.
[edit] Bulbourethral glands
- The bulbourethral glands produce a secretion for lubricating the male GU tract for ejaculation.
- The secretion of the bulburethral gland is clear and viscous.
[edit] Ejaculation sequence
- Ejaculation has a particular series of events regarding all these secretions:
- Bulbourethral glands discharge to lubricate.
- Prostate releases contents (via contraction of the fibromuscular stroma).
- Recall that the prostate's secretions serve to condition the female GU tract.
- The ductus deferens receives sympathetic stimulation to contract its muscularis (1->2 layers), thus pushing spermatozoa into the urethra.
- Seminal vesicles discharge their contents thus clearing the urethra by pushing semen distally.
- ID straight tubules by the presence of Sertoli cells.
- Ductus epididymis has very tall cells with cilia.
- Ductus deferens ID by stereocilia and three layers of muscle.
- Seminal gland: mucosal arches
- Prostate is ID'd by corpora amylacea (a concentration of glycoprotein-rich secretion in the lumen of the gland that stains eosinophilic).
[edit] Skin
[edit] Describe the basic histological structure of the skin


[edit] Identify the cell layers that constitute the epidermis
- Also, the granulosa and corenum layers are thicker in "thick skin".


[edit] Stratum basale
- The cells of the basal layer are connected to the basement membrane via hemidesmosomes.
3-Epidermal%20Layers.jpg
[edit] Stratum spinosa



[edit] Stratum granulosmum
- The granule-containing cells of the granulosum contain filaggrin, and intermediat filaments that help to form the tonofibrils along with
keratin.
- Note that these granules are called keratohyline granules.
- Kertohyline granules have no membrane.
- The cells of the granulosum are flattened polygonal cells--found in 3 to 5 layers.
- Just like "lamellae" in chloroblasts, lamellar granules have a sort of stacked-bags look
- Labellar graunules cannot be seen in light microscopy.
3363-63-6-1706-f06.gif


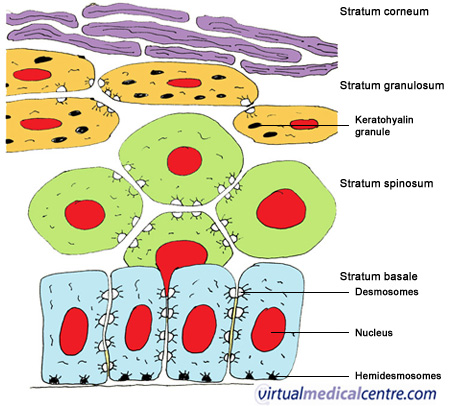
[edit] Stratum lucidum
- The lucidum (ironically) stains darkly.
[edit] Stratum corneum
1-Epidermal%20Layers.JPG
[edit] Dermo-epidermal junction
- Recall, too, that the epidermal cells of the basal layer are connected to the basement membrane via hemidesmosomes.
- The dermis contains lots of type 4 collagen.
- The superficial aspect of the dermis that is attached to the basement membrane of the epidermis is the lamina densa.
- The lamina densa of the dermis and the basement membrane of the epidermis are connected via anchoring filaments and anchoring fibrils.
- Note that anchoring filaments are composed of type 7 collagen.

[edit] Describe the cellular components of the epidermis and their functions
- There are four major cells of the epidermis: keratinocytes, melanocytes, langerhan cells, and merkel cells.
[edit] Keratinocytes

[edit] Melanocytes
- Melanocytes are derived from neural crest cells and function to generate the pigment melanin which protects cells from UV damage.
- Melanosomes are said to "mature" as they are produced; they turn from a light, circular shape to a dense cucumber shape.





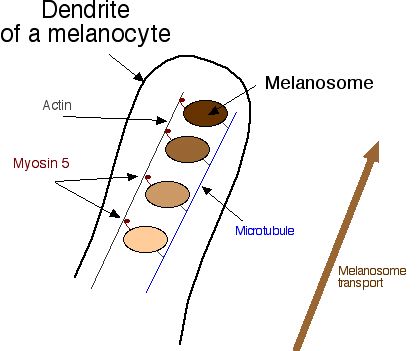

[edit] Langerhans cells
- Langerhans cells reside primarily in the spinosum layer (think "spines and chinese are for killing bad guys!).


[edit] Merkel cells
- Merkel cells (like melanocytes) are found primarily in the basal layer, which makes sense because they are a sensation cell that needs to be near a nerve
ending.
- Note that Merkel cells are found primarily in thick skin where touch needs to be highly sensitive.
- Merkel cells, along with the expanded terminal bulb of afferent, myelinated nerves, form the Merkel's corpuscle which detects touch as a
mechanoreceptor.
- The Merkel cells contain dense-cored neurotransmitter granules.

[edit] Describe the structural organization of the dermis
[edit] Papillary layer of the dermis
- Connective tissue of the papillary layer is composed of type 1 collagen, type 2 collagen, and elastic fibers.
[edit] Reticular layer of the dermis
- The reticular layer is composed of type 1 collagen and regularly oriented elastic fibers (called Langer's lines).





[edit] Identify other structures in the skin
[edit] Vessels
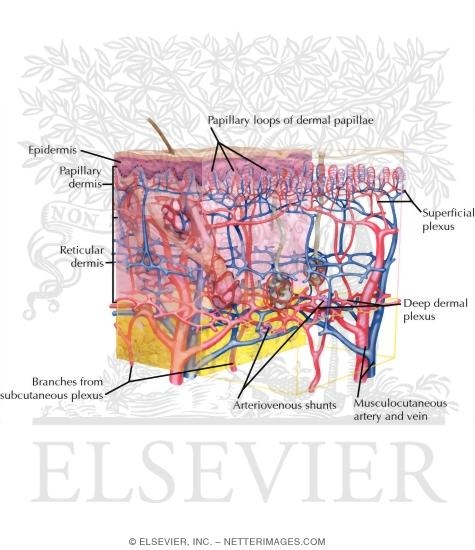

[edit] Sensory receptors
- There are four types of sensory receptors in the integumentary system (skin): free nerve endings, pacinian corpuscles, meissner's corpuscles, and ruffini's
corpuscles.
[edit] Free nerve endings
- Free nerve endings are found in the stratum granulosum and detect fine touch, heat, and cold.


[edit] Pacinian corpuscles
- Pacinian corpuscles are nerve endings surrounded by an oval encapsulation of connective tissue in the deeper dermis and hypodermis.
- Think maraca shaped and all that vibration!






[edit] Meissner's corpuscles
- Meissner's corpuscles are found in the papillary layer of the dermis and are sensitive to low frequency stimuli.
- Doesn't "meissner" sound like an old miser with a low, grumpy voice?
- Meissner's corpuscles are shaped like tapered mitochondria and are oriented perpendicular to the skin.





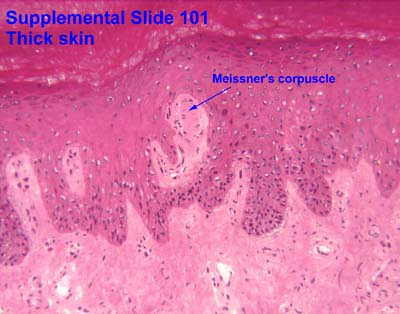


[edit] Ruffini's corpuscles
- Ruffini's corpuscles are simple mechanoreceptors and have an "elongated fusiform shape".

[edit] Sensory receptor images



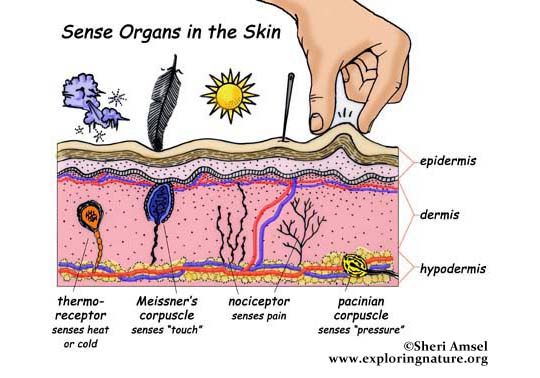
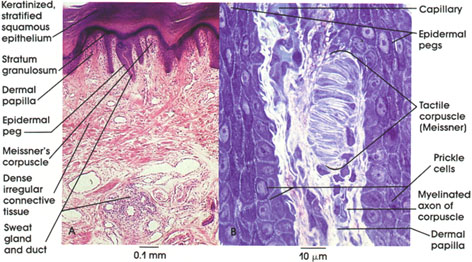


[edit] Hair follicles
- At the hair follicle a specialized layer called the glassy membrane (a type of basement membrane that is thickened and keratinized) separates the
epidermis and the dermis.
- During hair growth, the follicle has a bulbous end at the deepest part.
- Hair has three layers (from outside in): cuticle, cortex, and medulla.
- The cuticle is the outer most and is comprised of squamous cells.
- The cortex contains cuboidal cells that differentiate into keratinized cells.
- The medulla contains large cells with vacuoles that are moderately keratinized.
- Sebum is released into the infundibulum which is a pilosebaceous canal that surrounds the base of the growing hair.





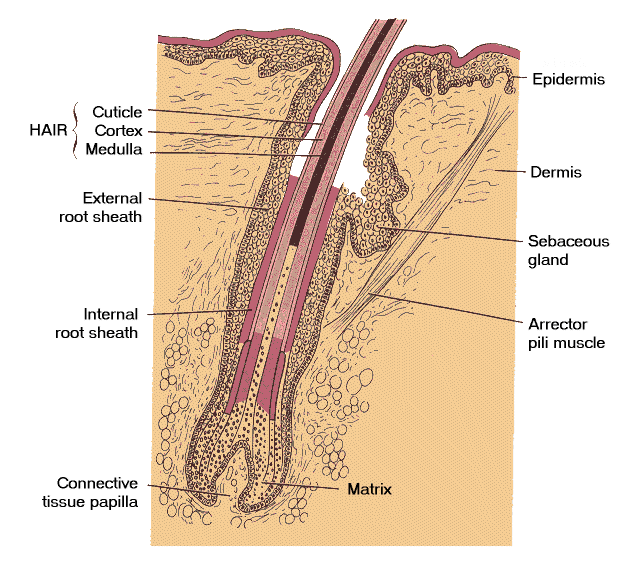


[edit] Phases of hair growth
[edit] Nails
- The nail plate sit in the nail bed which is formed by the stratum basale and spinosum.

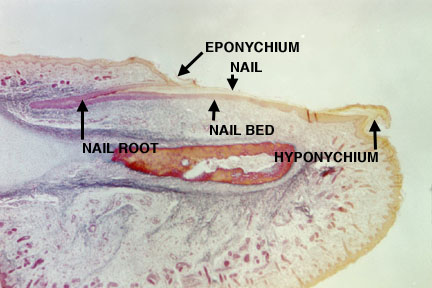


[edit] Glands

[edit] Sebaceous glands






[edit] Sweat glands
| Attribute | Merocrine | Apocrine |
|---|---|---|
| Secretion method | Merocrine | Apocrine and merocrine |
| Distribution | Widely distributed | Axillary and perineal regions only |
| Lumen size | Small lumen | Large lumen |
| Epithelial type | Stratified cuboidal | Simple cuboidal |
| Innervation | Cholinergic fibers (ach) | Adrenergic (cats) |
- Merocrine (eccrine):
- Apocrine:
- Visual differentiation:
- Lumen size: small = merocirne, large = apocrine
- Density: denser = merocrine, lighter = apocrine
- Surrounding cells: myoepithelial cells surround merocrine to help secrete, apocrine don't necessarily have surrounding cells.
[edit] Understand the mechanism of skin repair

[edit] Describe the histological findings in common skin diseases
[edit] Blistering
- Abnormalities at the epidermis-dermis junction are called bullous pemphigoid.
- Abnormalities of intercellular junctions are called pemphigus.
[edit] Psoraisis
- Psoriasis occurs when cells of the basal and spinosum layers demonstrate excessive proliferation and decreased cycle time which leads to increased
thickness.
- One can identify psoriasis by the presence of nuclei in the stratum corneum; this finding is called parakeratosis.



[edit] Skin cancer

[edit] Basal cell carcinoma





[edit] Squamous cell carcinoma


[edit] Malignant melanoma

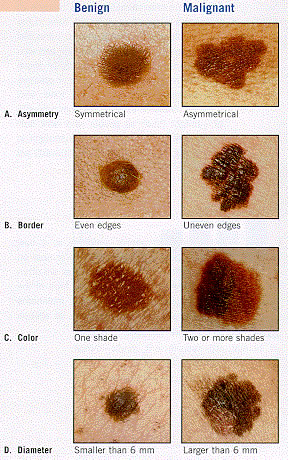


[edit] Griscelli syndrome
- Griscelli syndrome can result from a defective Rab27a protein which is part of the transport complex that moves melanosomes along microtubules
for cyotocrine passage to other cells.
[edit] The eye
[edit] Optical anatomy

[edit] Wall of the eye

[edit] Eyeball function by component

[edit] Cornea
- The layers (superficial to deep): epidermis, Bowman's membrane, stroma, Descemet's membrane, and endothelium.
- With age, Descemet's membrane decreases in transparency and leads to decreased light transmission.

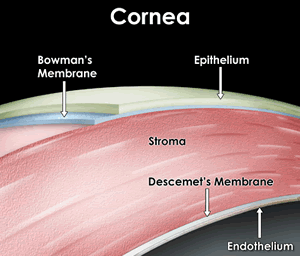


[edit] Epithelium of the cornea
- The epithelium of the cornea is made of stratified, squamous, non-keratinized epithelium.



[edit] Stroma of the cornea
- The stroma is series of layers of fibrocytes, proteoglycans, and ECM fibers with alternately oriented collagen fibers.
- Note that collagen of the stroma is of type 1 and 5 and are non-fibrillar.
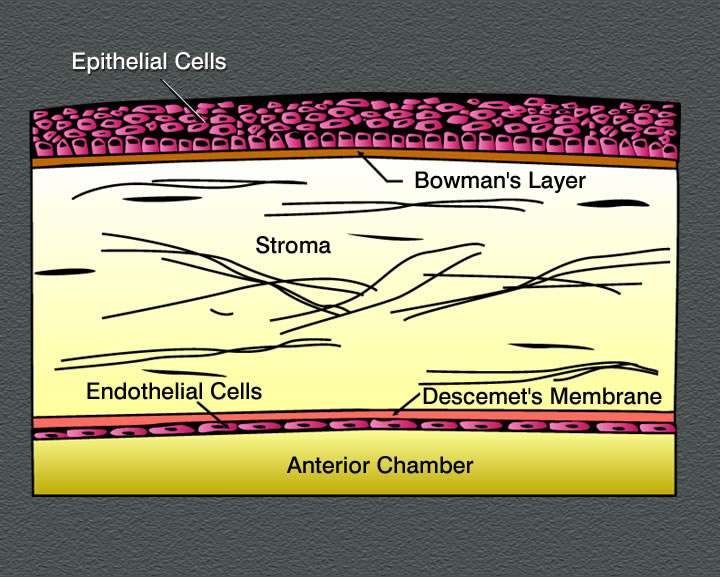
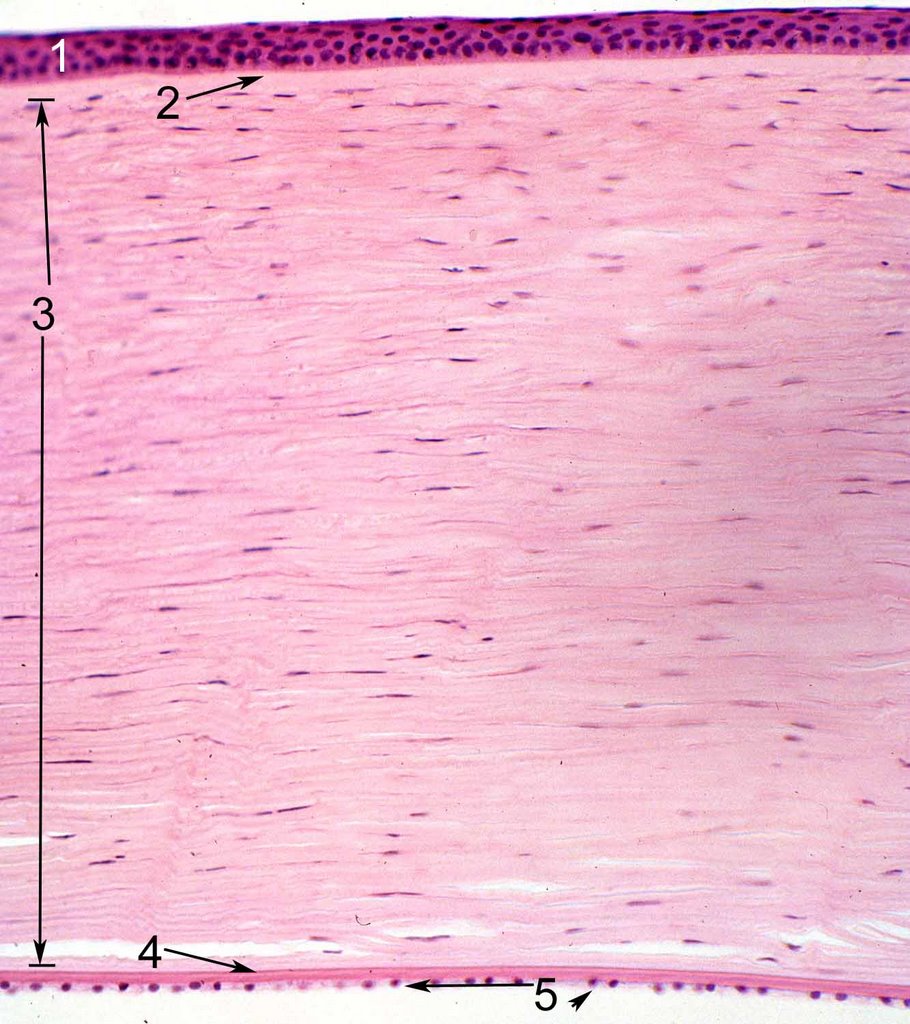

[edit] Endothelium of the cornea

[edit] Uvea

[edit] Choroid of the uvea
- The choroid is highly pigmented and found between the sclera (part of the tunica fibrous) and the retina.
- The choroid has three layers: sclera vasculature, retinal vasculature, and Brunch's membrane.
- The retinal vasculature layer is also called the choriocapillary layer.
- Bruch's membrane is also sometimes called a glassy membrane.

[edit] Ciliary body
- Far vision requires a flattened lens (think flat like a frisbee which you hope will go "far") so the ciliary muscle relaxes, the zonules tense, and the lens
is pulled into a flatter shape.
- Near vision requires a bulged lens so the ciliary muscle contracts, the zonules relax, and the lens relaxes into a bulge.
- Oxytalin fibers are used to attach the basement membrane of the non-pigmented epithelium of the ciliary body (part of the uvea) to the basement
membrane (called the lens capsule) of the lens.

- Ciliary processes:
- Aqueous solution has little protein, some glucose, and similar ion concentration as plasma.
- The pigmented layer (deep layer) of the ciliary process is continuous with the pigmented layer of the retina.
- The non-pigmented layer's (secretory, surface layer) apical surface faces the pigmented layer and the basolateral surface has lots of folds and borders
the posterior chamber.
- We call the space between the pigmented and non-pigmented cells the ciliary channel and consider it a potential space.
- Blood-aqueous barrier: occluding junctions at the apex of the ciliary process's surface epithelium keeps blood and aqueous solution from
mixing.


.jpg)
[edit] Iris
- Note that the posterior epithelium is one and the same as the myoepithelium: it has muscle fibers in it and is responsible for dilating the pupil.
- The malanocytes of the stroma determine eye color.
- The stroma is responsible for constricting the pupil.
- Note that dilation is sympathetic and contraction is parasympathetic.
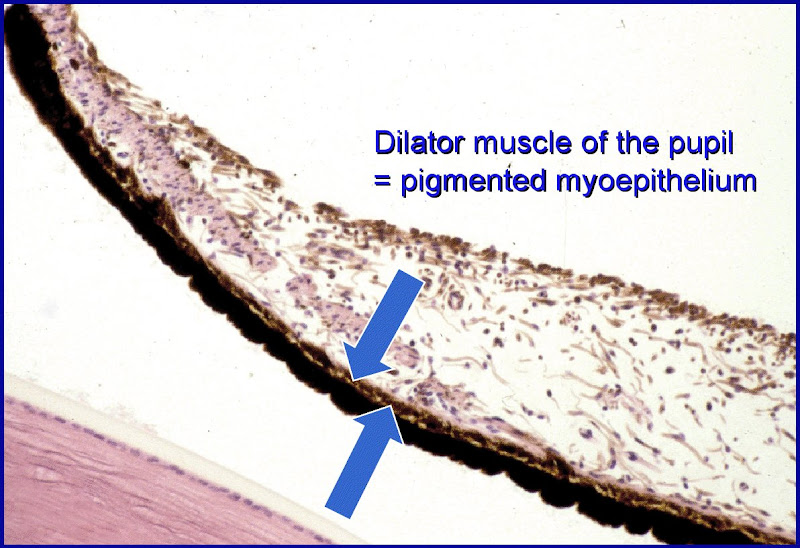
[edit] Eye color
[edit] Lens
- The epithelium on the anterior side of the lens is simple cuboidal.
- Note that the lens epithelium does not have occluding junctions.

- In the case of cataracts, lens fibers (the cells that span ant-post and have crystallin) turn opaque and refract light poorly.
- In the case of presbyopia, the lens loses elasticity (some loss is normal during aging) such that the pt cannot accomadate well and thus has
poor near vision.
[edit] Retina
[edit] Pigmented epithelium of the Retina
- The pigmented epithelium of the retina is simple cuboidal and is adjacent to the inner-most layer of the uvea (Bruch's membrane).
- Note that secretion is enabled via Na-K ATPase.
- concentrates and estrifies vitamin A for easy reproduction of rhodopsin by rods
[edit] Neural retina


- Rods versus cones
- Rods use rhodopsin while cones have three distinct pigments for three colors: red, green, and blue.
| Attribute | Rods | Cones |
|---|---|---|
| Function | Night vision (scotopic), low acquity | Day vision (photopic), color, high acquity |
| Photopigment | Rhodopsin | 3 pigments, 3 wavelength specificities |
| Distribution | "Peripheral" | "Central" |
| Population count | 120 million | 6 million |