Astrazeneca PLC
From Lauraibm
Contents |
MI Summary
Climate Change
In common with most businesses, our potential impact on climate change arises from the greenhouse gas emissions from energy use at our facilities, from other in-house activities and from the various means of transport we use. However, we also face an additional challenge since some of our asthma therapy products use propellant gases that potentially contribute to ozone depletion and global warming. In recent years, we have been making good progress in reducing our emissions but our challenge has always been to sustain improvement as we continue to grow our business.
We have identified areas of our business where further improvements can be made to reduce our emissions of global warming gases. These include, amongst other things:
- Implementation of further energy conservation programmes, particularly related to fume cupboards in laboratories.
- Implementation of green technology principles in our process design.
- Further investment in greener energy supply from external power suppliers.
- Installation of additional combined heat and power plants.
- Investment in ‘cleaner’ vehicles.
Nevertheless, our major challenge continues to be reducing these emissions quickly enough to offset the impact of our growing business. We will continue to work hard to manage our impact, and our new climate change target aims to ensure that our absolute emissions in 2010 will be no greater than they were at the start of the decade and 40% less than they were in 1990. Although the greenhouse gas emissions from our business operations will continue to fall, as a result of the planned launch of Symbicort pMDI in 2007, we will not be able to continue to achieve the reductions of total greenhouse gases (including emissions from products) that we have delivered each year since 2000.
We are committed to achieving our 2010 target without compromising our ability to provide new inhalation therapies that bring benefit for patients. Therefore the climate change objectives approved by the AstraZeneca Board in 2005 require very substantial efforts to be made across our business to produce, by 2010, an absolute reduction of 12% in global warming emissions from all sources other than pMDIs, when compared with 2005.
(1)
Global Warming
Emissions from Energy Use
We use energy to manufacture our products and to heat, cool and light our facilities. Using fossil fuels, either directly or to generate electricity, results in the emission of CO2.
Different energy sources give rise to different levels of CO2 emissions. For instance district heating, primarily used in Nordic countries, normally uses bio-fuel, a renewable energy source, and results in very low net CO2 releases compared with burning coal, oil or gas. We follow the GhG Protocol guidelines to calculate our emissions. Specific emission factors for direct combustion of fuels are taken from the IPCC (Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories, Workbook, Volume 2), and those for purchased electricity are sourced from the International Energy Agency ( www.iea.org.)
Third parties also undertake some of our manufacturing and we have been making efforts to understand what emissions may come from this activity. See the section on Sustainable production for further details of this exercise.
Our approach Although AstraZeneca does not use a large amount of energy relative to its size, we recognise the importance of implementing programmes to maximise efficiency and minimise emissions.
Some progress is being made at a corporate level, such as building a proportion of renewable energy into energy procurement contracts and large capital programmes such as Combined Heat & Power (CHP) or cogeneration schemes. In addition, many of our sites around the world are pursuing energy efficiency projects and have made significant improvements.
Traditional generators burn fuel to provide a jet of hot gases. These are used to spin turbines that generate the electricity. But they also waste much of the energy content of the fuel as the exhaust carries a lot of heat, to be lost up a chimney. CHP uses this “waste” energy in the exhaust gases to generate steam and this means that the fuel efficiency is doubled compared to traditional turbine generation plant. CHP plant has been installed at two of our key manufacturing sites in Macclesfield, UK and Canovanas, Puerto Rico.
The market for renewable electricity remains volatile as the growth in demand far outstrips increases in supply capacity. Nevertheless, our take-up of electricity from renewable sources has exceeded our expectations since we secured a three-year supply contract with nPower to provide the majority of our UK facilities with Climate Change Levy exempt electricity for three years from July 2004. Furthermore, our headquarters building in London, our environmental science facility in Devon and our large research site in Leicestershire now have their electricity needs supplied from guaranteed CO2-free renewable resources. 39 kte CO2 has been avoided by these measures.
Our performance
During 2006, total energy use at our facilities was 2460 GWh; the same as 2005 consumption. The main energy sources were natural gas (49%) and electricity (37%). The energy consumed in manufacturing activities accounts for two thirds of the total. The Group's energy costs are approximately $150 million. CO2 emissions from energy in 2006 were 568 kte, an increase of 2% from 2005. The nature and scale of our energy use and emissions of CO2 are shown in the charts.
Emissions from Transport
Emissions from transport have an impact on climate change, acidification and photochemical smog. In a complex global organisation such as AstraZeneca it is difficult to produce precise data for emissions from transport. Furthermore, different calculation methods for estimating acidification and photochemical smog give very different results and we therefore only report our emissions of CO2. We use the GhG Protocol guidelines to calculate transport emissions.
Our approach We have selected a few logistic and road haulage companies to partner for our main distribution routes. Priority is given to companies who have good procedures for SHE and quality management, modern trucks with efficient engines and drivers trained in eco- and safety driving. Concerning airlines, the age and type of their fleet is taken into consideration in the selection of partners.
A reduction in the demand for freight transport can be achieved by either reducing the volume of goods or reducing the distance for movements. Bulk transport and final packing of our products at our marketing companies reduce our demand for freight. Internally, efforts have been made to make transport more efficient and to use more environmentally friendly packaging options. The special handling aircraft shipment, using slip-sheet techniques, reduces volume significantly. Normal handling would require pallets, which have a much bigger volume than the slip-sheets. Wherever possible, reusable blankets have replaced polystyrene boxes for temperature-controlled transport.
Ecopar, a synthetic diesel made from natural gas is used at AZ sites in Södertälje, Sweden. It was trailed in tractors and machinery used for out-door maintenance work on site last year. The outcome was positive and it was decided to continue the use and to look for other areas to use Ecopar and by this to support the supplier’s development of synthetic diesel from renewable resources such as silage or organic waste products.
AstraZeneca’s sales forces have tested bio-ethanol powered cars and hybrid cars. From 2005 and onwards, all new cars purchased for the sales force in Brazil can be refuelled with ethanol 'Flex Fuel'. Today more than 96% of the cars in the Brazilian Marketing & Sales’ car fleet can be powered by either ethanol or petrol. Ethanol is a non-fossil fuel from renewable resources and has much lower impact on Climate Change than equivalent use of petrol.
As part of our 2006-2010 SHE objectives, functional groups across US Operations are collaborating to reduce the energy use. US Fleet Services is making progress on our target to reduce greenhouse gas emissions in our vehicles 12% - about 9,000 tonnes - by 2010. To accomplish this goal, we are selecting new vehicles that reflect an eco-friendly approach to the company's driving needs. For example, all new vehicles obtain, on average, 25 miles per US gallon. (9.4 l/ 100 km) Thanks to a successful pilot program earlier this year, which used partial-zero emissions hybrid vehicles at 21 locations across the US, new drivers in metropolitan areas are now able to choose a hybrid vehicle. Hybrid cars are fuel-efficient and have decreased, if any, exhaust emissions at low speed and when idling. We now have 29 hybrids in the fleet, and we are looking forward to expanding this number in 2007. The careful selection of vehicles, combined with existing programs to maximize fuel efficiency by maintaining vehicles properly and training drivers in fuel-efficient behaviours, has put us on the fast track to achieving our emissions reduction goal.
10 SAAB Bio-power was tested in Sweden during the winter 2005-6. The result was positive and by end of 2006 there were 50 'Flex Fuel' vehicles in the Swedish fleet. These vehicles can be powered by either petrol or E85 (85% ethanol and 15% petrol.) Our 50 ‘Flex Fuel’ cars have the potential to deliver a reduction in CO2 emissions each year of around 200 tonnes. We also provide our drivers with training in “eco-driving” techniques, which encourages them to think ahead, planning acceleration and deceleration, anticipating traffic flow and maintaining a steady speed to improve fuel efficiency as well as safety.
Local initiatives in Brazil, the UK and Sweden are in place to encourage more energy-efficient "green" commuting. Web-based tools for car-share are in place in for all employees Sweden and Alderley Park, UK are in use used to match people for commuting, business travel and private trips. Green Commuter Plans have been established in Sao Paolo, Brazil and Alderley Park, UK, where the company supports bus-links designed to match the commuting habits of our employees. In Brazil many employees commute by bus to the AZ site. There are nine bus journeys during the main shift each day and four additional trips that assist the company during the other shifts. Approximately 280 employees use this system to travel between work and home. In Sweden the travel pattern to work has been mapped and this survey will guide in encouraging more environmentally friendly commuting behaviour. AstraZeneca in Delaware in the US received the Best Workplaces for Commuters Race Excellence Gold award and is committed to working with their employees to promote non-single occupancy vehicle travel and reduce traffic congestion in the Wilmington area. Also, our Massachusetts site has received recognition as a Best Workplace for Commuters. Best Workplaces is a part of the EPA.
Our performance
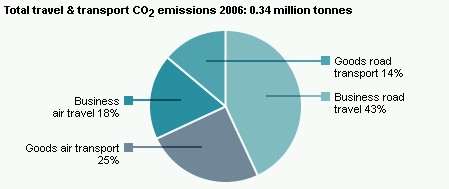
During 2006, CO2 emissions from transport activities, including transport of products and business travel by road and air, totalled 0.34 million tonnes. The biggest single contribution to these emissions is associated with business car travel for sales and marketing activities. Our growing business makes travel-related reductions an ongoing challenge.
In 2006, business air travel contributed 61 thousand tonnes to our CO2 emissions, an increase of 4% from 2005,though effective use of video and teleconferencing is helping limit the need for our personnel to travel.
During 2006 the reported business travel by car amounted to 703 million kilometres, a distance equivalent to 17,600 times around the world. This is an increase on last year of 8% and is mainly attributable to an increase in sales and marketing activity and improved reporting. More than 90% of the miles driven are associated with sales and marketing
(2)
Ozone Depletion
Although we have a programme to eliminate their use, we still have residual amounts of chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs) and halons in fire protection and refrigeration systems. In addition, AstraZeneca currently uses some substances that contribute to ozone depletion, primarily in connection with metered-dose inhalation products prescribed to asthmatics. However, we are working to reduce our use of these materials by substitution and through innovative new product designs that do not require propellant gases.
CFCs, HCFCs and HFCs CFCs and HCFCs are known to be capable of depleting the ozone layer, but they are also very potent 'greenhouse gases'.
Hydrofluorocarbons (HFCs) have in general lower Global Warming Potential (GWP) compared to the CFCs and HCFCs and have no potential to deplete the ozone layer.
Our emission of Ozone Depleting Substances (ODP) falls into two main categories:
- Inhalation products By far the largest component of our ODP emissions is from patient use of our Metered Dose Inhalers. Information on this issue can be found in the Products & Packaging section.
- Use at facilities
A much smaller fraction of our ODP emissions, less than 1%, arises from our use of these substances at facilities, primarily in connection with the manufacture of inhalation medicine products and in air conditioning, cooling and refrigeration. While current emissions are extremely small, in accordance with our policy on refrigeration, we are gradually replacing all CFCs and HCFCs used in refrigeration with gases that have no ODP and substantially lower GWP. For example, a local phase-out programme at our manufacturing site in Porrino, Spain, resulted in replacement of HCFC-22 as a refrigerant with a non-ozone depleting HFC alternative.
12.4 tonnes of CFCs, 3.0 tonnes of HCFCs, and 32.1 tonnes of HFCs were used at our facilities during 2006. (Third parties manufacture some of our pMDI products and their use of CFCs is not included). AstraZeneca's use of these compounds has increased from last year; 7.8 tonnes CFCs 2.1 tonnes of HCFCs, and 11.5 tonnes of HFCs were used in 2005.
Emissions in 2006 were 0.1 tonnes of CFCs, 2.0 tonnes of HCFCs and 4.2 tonnes of HFCs. The ozone-depleting potential (ODP) of all the substances emitted from facilities in 2006 was equivalent to 0.25 tonnes of CFC-11. The majority of this emission arises from the manufacture of metered dose inhalers for the treatment of respiratory disease.
(3)
Sustainable Production
Products and Packaging
Products We recognise that the potential impact of our products on the environment can occur throughout the lifecycle of the materials from which our products are made. The risk of adverse impacts can normally be reduced or eliminated by considering environmental issues carefully during the product development process. However, pharmaceuticals generate major challenges in this respect, since there is usually very limited flexibility to modify the active molecule to improve its environmental profile whilst preserving its efficacy and minimising any potential side effects.
We are continually working to improve the way environmental issues are considered in process development, asset design and marketing by developing procedures that continuously improve the integration of SHE considerations into the product development process, based, in part, on whole lifecycle techniques
Packaging Packaging material is a key area on which we continue to focus. Robust packaging from a quality and supply perspective is necessary to ensure that the product reaches the patient in a safe and secure manner. The development of functional packaging with minimal resource consumption remains a challenge.
In Europe, there is often a requirement to distribute pharmaceutical tablets packaged in 'blister packs'. The conventional packaging material used is polyvinylchloride (PVC), which, along with the related polymer polyvinylidine chloride (PVdC), is one of the most used materials due to its appropriate characteristics. Scientific, technical and economic questions have been raised concerning PVC and its effects on human health and the environment but there is still no scientific consensus on the answers to these questions. However, to reduce emissions resulting from disposal of packaging waste in Japan, major pharmaceutical companies have voluntarily changed their blister packaging materials. AstraZeneca has exchanged PVC material with polypropylene (PP) blister packaging in Japan for all tablet products for the local market and transferred the local Swedish product Alvedon to PP mono blister packaging.
We continually look for opportunities to use more environmentally friendly and ergonomic packaging materials and practices to minimize the solid waste stream from the package development and commercial packaging processes.
In the US, AstraZeneca was selected by Food and Drug Packaging Magazine as the 2005 "Drug Packager of the Year" award winner for several innovative new package launches and new high speed packaging processes. Our manufacturing facility in Spain is working on several small projects which will reduce the quantity of waste associated with sales products and manufacturing waste. Our facility in Gärtuna in Sweden is one of the world's largest formulation plant manufacturing tablets for a global market. A reduction in the use of packaging material will be achieved by optimising the current tablet bulk box used for shipping most tablets. The implementation of this project started towards the end of 2005. After implementation approximately 20 tons of cardboard and 5 tons of aluminium bags will be eliminated from the bulk packaging on a yearly basis.
(4)
Waste Management
AstraZeneca manufactures pharmaceutical products from a mainly synthetic chemistry base. The amount of waste our operations generate is affected by a variety of factors. This includes the level of production and the volume of process wastewater treated at our facilities. Waste is categorized as ‘hazardous waste’ or ‘other waste’ according to national legislation, which varies in its definitions. The majority of our hazardous waste consists of solvents and process wastewater from the production of pharmaceuticals. Other waste includes general waste and sludge from on-site wastewater treatment plants.
We aim to use materials efficiently, maximise recycling and, where possible, avoid the use of hazardous substances.
The primary objective in waste minimisation is waste prevention. Where this is not practicable, we strive toward the re-use and recycling of materials, including energy recovery. Programmes designed to reduce the amount of waste we generate include the continual improvement of existing production processes and the better design of new ones, improved purchasing processes and internal waste awareness rogrammes.
Third parties also undertake some of our manufacturing. Although their waste data are not included in our reported figures, we continue to track and report third party waste and energy consumption in the Sustainable Production section.
Performance
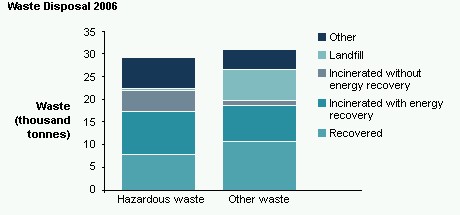
The amounts of hazardous and other waste that we generated between 2001 and 2006 are shown in the table below. Hazardous waste produced reduced markedly between 2001 and 2002 as a result of improvements to processes and reclassification of some waste streams. Although sales have increased by 30% since 2003, the AstraZeneca sites have been able to improve efficiency in order to maintain the quantities of hazardous waste that need to be disposed of at approximately the same level. Since 2001 the amount of other waste has increased by a small amount but this also remains well below the increase seen in sales.
Waste disposal routes for our hazardous and other waste in 2006 are shown in the chart below. We differentiate between the material recycled and the waste incinerated with energy recovery. It should be noted that these figures do not include the very large amount of material reused and recycled within our facilities, such as solvent recovery and reuse, since this material is excluded from our definition of waste.
The amount of hazardous waste recovered (including material recycling and energy recovery) was 59%.
Total waste recycling rates in 2006 were 60% (5)
Emissions to Air & Water
Air
Information regarding emissions to air associated with Global Warming and Ozone Depletion are discussed in other sections.
Volatile Organic Compounds (VOCs) are released to air primarily from the use of solvents in manufacturing processes and research and development activities. Emissions of VOCs to air can give rise to ground level ozone due to the action of UV light. The effects of ground level ozone act at a regional level. Elevated levels may be a health hazard and have an adverse effect on plant life. Some VOCs are also greenhouse gases and may contribute to climate change. VOCs can be divided into two categories; non-halogenated VOCs that account for the majority of our use and halogenated VOCs that are generally considered to have a higher environmental impact.
Solvents are recycled and re-used as much as possible at our main manufacturing facilities to minimise the amounts of fresh solvent consumed. We also minimise emissions to air by using VOC abatement equipment including technologies such as catalytic oxidation, low-temperature cryogenic condensation and adsorption of pollutants on activated carbon.
Performance Both total solvent use and related VOC emissions have decreased slightly from 2005 to 2006. The fraction of VOCs emitted remains less than 2% of the quantity consumed.
Halogenated solvent use (443 tonnes) and related VOC emissions (23 tonnes) make up a small part of these totals.
Water
All our facilities use water and subsequently discharge wastewater to be treated either on site or by local treatment authorities. We measure the total volume we use and the load that our effluents from manufacturing place on the aquatic environment. See the Biodiversity, land and water use section for more details about our water use.
Effluent treatment is being upgraded at several of our manufacturing facilities as part of our process of continuous improvement. Consistent with our commitment to product stewardship, we are pursuing efforts to further minimise the amounts of any of our products being released into the environment in effluent discharges from our facilities.
Performance Discharged organic material from our manufacturing sites expressed as Chemical Oxygen Demand (COD) increased by 1.4 % compared to the previous year and amounted to 221 tonnes.
(6)