Acid-base balance
From Iusmphysiology
Revision as of 18:28, 19 April 2011 by 24.15.60.132 (Talk)
- started here on 03/30/11.
[edit] Acid-base balance
[edit] Objectives
- Define the following: acid, base, buffer, pH. Give the normal range of arterial blood pH and the limits compatible with life. Explain why constancy of pH is important.
- State the isohydric principle. List the important chemical buffers present in extracellular fluid, intracellular fluid, and bone.
- Write the Henderson-Hasselbalch equation for the bicarbonate/CO2 system. Write Henderson's equation for calculating [HCO3-] from [H+] and PCO2 measurements.
- Explain why the bicarbonate/CO2 system is so important.
- List the four simple acid-base disturbances. Describe for each: 1) the primary defect, 2) changes in arterial blood chemistry (pH, PCO2, and plasma [HCO3-]), 3) some common causes, 4) chemical buffering processes, and 5) respiratory and renal compensations.
- Given plasma electrolyte concentrations, calculate and interpret the anion gap.
- Given values for arterial blood pH, plasma [HCO3-], and PCO2 (or any two of the three), be able to identify the type of acid-base disturbance present.
[edit] Normal pH in arterial blood
- A normal pH is 7.4 (7.38-7.42), at which point the concentration of H+ is 40 (38 - 42) nmol / liter.
- Survivable pH is 7.0-7.6 which is considered acidosis and alkalosis, respectively.
- Note that this is a four fold change in H+ concentration.
- Death by change in pH occurs as a result of the changes in intracellular proteins upon change in pH.
[edit] Threats to pH of extracellular fluid
- There are two major forces that affect extracellular pH: oxidative phosphorylation and protein metabolism.
- Both of these processes produce sources of acid: oxidative phosphorylation produces CO2 and protein metabolism produces H2SO4 and HCl.
- Oxidative phosphorylation as a source of extracellular acid:
- Recall that oxphos dumps electrons onto oxygen and secretes this waste as CO2.
- Recall that CO2--via carbonic anhydrase--affects the extracellular levels of H+ and HCO3-.
- That is, as CO2 rises, more H2CO3 and H+ are found in the blood; the pH decrease.
- We say CO2 is a volitile acid because it indirectly affects H+ levels.
- 13-20 moles of CO2 are produced each day by oxphos.
- Protein metabolism as a source of extracellular acid:
- Recall that proteins are digested for energy and as a source of amino acids.
- Metabolism requires the removal and storage of the many hydrogens on the proteins and thus can generate H+.
- Met or Cys metabolism generates H2SO4.
- Lys or Arg metabolism generates HCl.
- We say protein metabolism generates fixed acids because it directly affects H+ levels.
- 40-60 moles of fixed acid are produced each day by protein metabolism.
[edit] Chemical buffers
- Recall that a chemical buffer is an acid (HA) and it's conjugate base (A-).
- There is an equilibrium among these two components as the hydrogen dissociates and reassociates: HA <-> H+ + A-
- The Ka describes the balance between the two entities (A- and HA): Ka = [H+][A-] / [HA]
- Recall the Henderson-Hassalbach equation which describes the pH in terms of the Ka and the amount of conjugate base (A-) present in a sample: pH = pKa + log([A-] / [HA])
- Recall that pKa = log(Ka) = log([H+][A-] / [HA])
- So the Henderson-Hasselbach equation takes the (log of the) expected balance ([H+][A-] / [HA]) and modifies it by adding (the log of) the ratio of conjugate base to conjugate acid.
- Chemical buffers of the body come exist in three compartments: ECF, ICF, and bone.
- Each of the buffers in these three compartments can add or remove H+ from the system in order to buffer the pH.
- The ECF contains HCO3- / CO2, plasma proteins, and inorganic phosphates.
- The ICF contains proteins, organic phosphates, some HCO3- / CO2.
- The bone contains pohosphate and carbonate salts.
[edit] Isohydric principle
- The isohydric principle says that at any given concentration of H+, all conjugate acid / base pairs are in equilibrium.
- This means that the pH can be determined from any pair of conjugate acid / base pairs using their pKa.
- pH = pKa + log([A-] / [HA])
- pH = 6.1 + log([HCO3-] / 0.03*PCO2)
- pH = 6.8 + log([HPO4] / [H2PO4])
- pH = pKHPr + log([Pr-] / [HPr])
- pH = pKa + log([A-] / [HA])
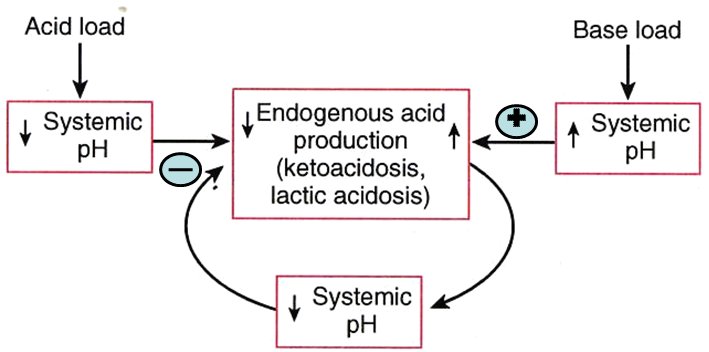
[edit] Negative feedback controls on endogenous acid production
- There is a negative feedback system that helps resist changes in systemic pH when endogenous acid production is high.
- This system may have an effect during vigorous activity or diabetic ketoacidosis.
- This negative feedback system is based on cellular enzymes that produce endogenous acid (think back to oxphos and protein metabolism as endogenous sources of acid).
- Named examples of acid-producing processes include ketoacidosis and lactic acidosis.
- Negative feedback of pH change is probably mediated by the inherent effect that a change in pH has on these enzymes that make endogenous acid: as as pH goes up, endogenous acid decreases (because the enzymes don't function as well at high pH) and as pH drops endogenous acid increases (because the enzymes work better at higher pH).
[edit] The importance of the HCO3 / CO2 buffer system
- The HCO3 / CO2 buffer has two characteristics that make it very effective: abundance of buffer pair molecules, an "open" system.
- Bicarb / CO2 is a good buffer because of it's abundance:
- There is 24 mM of HCO3 in the blood.
- There is 1.2 mM of CO2 in the blood and 13-20 moles produced each day.
- Having lots of molecules means that many molecules of H+ can be bound or unbound as needed.
- Bicarb / CO2 is a good buffer because it is an "open" system:
- Recall that CO2 can be breathed off at the lungs and HCO3 can be secreted by the kidneys.
- Because of these physiological connections to organs that "open" up to the external environment, the HCO3 / CO2 system is considered "open".
- Note that the lungs can respond quickly (within minutes) and the kidneys respond slowly (within days) to balance HCO3 / CO2.
[edit] HCO3 / CO2 equilibrium
- Recall the carbonic anhydrase driving equation: CO2 (aq) + H20 <-> H2CO3 <-> H+ + HCO3
- Recall that the amount of aqueous CO2 is always 0.03 * PCO2.
- This is useful because PCO2 is measurable in the clinical setting.
- When PCO2 is 40 mmHg (pretty normal), CO2 (aq) is 1.2 mM.
- So when we calculate pH based on this:
- pH = pKa + log([A-] / [HA])
- pH = 6.1 + log([HCO3 / [CO2])
- pH = 6.1 + log([HCO3 / 0.03 * PCO2)
- pH = 6.1 + log(24 / 1.2)
- The 20:1 ratio of HCO3 : aqueous CO2 is key to having a normal pH.
- pH = 6.1 + log(20)
- pH = 6.1 + 1.3
- pH = 7.4
- But there is a simpler way: the clinical equation.
- In clinical settings, logs are not really practical so we use an alternative equation.
- The clinical equation uses two values easily procured by labs (PCO2 and [HCO3]) to calculate the concentration of H+ ([H+]) which is compared to an expected value (40 nM) to determine if the pt is acidotic ([H+] higher than 40 nM) or alkalotic ([H+] lower than 40 nM).
- [H+] = 24 * PCO2 / [HCO3]
- Recall that PCO2 and [HCO3] are usually known so we are calculating to find [H+].
- We have an expectation as to what a normal [H+] should be (40 nM) and this equation tells us if [H+] is high (low pH, acidosis) or low (high pH, alkalosis).
[edit] The lungs as an opening to the environment
- Recall that the lungs can breath off CO2 and thus decrease the pH of the blood (shifts equation away from H+ + HCO3-).
- As ventilation increases, more CO2 is breathed off.
- Ventilation is stimulated by increased arterial PCO2 or decreased pH.
- Note that neither of these forces can cause at pt to ventilate to their full voluntary ventilation capacity; thus, one can hyperventilate themselves into passing out--because one can throw off acid-base balance by ventilating too rapidly so the body arrests auto-hyperventilation by blacking out.
[edit] 4 acid-base distrubances
- There are four acid-base distrubances: acidosis and alkalosis arising from respiratory or metabolic origins.
- Recall that there are three compensation mechanisms that are very fast, fast, and slow: chemical buffering of the blood by HCO3 / CO2 (very fast, seconds), respiratory compensation by breathing off CO2 (fast, minutes), and renal compensation by excreting H+ / HCO3 (slow, days).
- Note that in respiratory acid-base imbalances, the primary variable that has changed is CO2 (because the lungs can only increase or decrease this one variable).
- Therefore, compensation mechanisms in pulmonary malfunction will always attempt to change HCO3 in the same direction as CO2.
- Note that in metabolic acid-base imbalances, the primary variable that has changed can be H+ or HCO3- (because metabolic processes can cause a change in either acid or base production).
- Therefore, compensation mechanisms in metabolic malfunction can attempt to change the opposite factor (base in an acid-malfunction or acid in a base-malfunction) in the same direction or can attempt to counteract the changes to CO2 levels.
[edit] Respiratory acidosis
- Respiratory acidosis is defined as "any abnormal pulmonary function that results in CO2 accumulation".
- As CO2 accumulates the equation shifts to the right, toward H+ and HCO3.
- However, HCO3 production does not keep up with the shift so H+ > HCO3-.
- Common cause: hypoventilation
- Compensation:
- Recall that compensation mechanisms attempt to normalize the HCO3 / CO2 ratio by moving the opposite factor in the same direction; therefore as CO2 increases, the compensation mechanisms will attempt to increase HCO3, also.
- Chemical buffering: mostly achieved by proteins binding H+ in cells (think Hb).
- Pulmonary compensation: none, lungs are the problem
- Renal compensation: raise the HCO3 content of the blood; attempt to restore a HCO3 / CO2 ratio of 20:1.
- This is an attempt to modify the HCO3 / CO2 ratio by increasing HCO3.
- Acute respiratory acidosis results in a renal compensation of about 1 mEq / L of HCO3 production for every 10 mmHg increase in PCO2.
- Chronic respirartory acidosis results in a renal compensation of about 4 mEq / L of HCO3 production for every 10 mmHg increase in PCO2.
[edit] Respiratory alkalosis
- Definition: "any abnormal pulmonary function that results in CO2 deficiency".
- As CO2 is released, the equation shifts to the left, decreasing H+ and leaving HCO3 to cause alkalosis.
- Common causes: hyperventilation
- Compensation:
- Recall that compensation mechanisms attempt to normalize the HCO3 / CO2 ratio (to 20:1) by changing the non-effected value in the same direction.
- In respiratory alkalosis, the CO2 level is too low so the body attempts to compensate by decreasing the amount of HCO3-.
- Chemical buffering: mostly achieved by release of H+ by intracellular proteins.
- Pulmonary compensation: none, lungs are the problem.
- Renal compensation: decrease the plasma HCO3- levels
- decrease HCO3- production, increase HCO3- secretion
[edit] Metabolic acidosis
- Definition: "any abnormal function that results in a gain of acid or loss of base (excepting the gain of H2CO3)".
- Recall that while pulmonary acid-base imbalances result from changes in CO2,] metabolic acid-base imbalances can result from acid or base changes.
- Adding an acid makes the pt acidotic and pushes the HCO3 / CO2 reaction toward CO2.
- Removing a base makes the pt acidotic and pulls the HCO3 / CO2 reaction toward H+ / HCO3-.
- Common causes:
- renal failure,
- excessive intake of nonvolatile acids,
- excessive production of nonvolatile acids (ketoacidosis, lactic acidosis, ingestion of acidosis, ingestion of acidifying agents),
- poisons (salicylate, methanol, ethylene glycol),
- loss of bicarbonate (excessive urinary excretion of bicarbonate [renal tubular acidosis], diarrhea)
- Compensation:
- Chemical buffering: half of the buffering occurs in the cells and bone, HCO3- is the main ECF buffering base.
- Pulmonary compensation: prompt hyperventilation to lower the PCO2; cannot completely compensate, however.
- Note that in the case of added acid, this would breathe off the elevated CO2, decreasing acid.
- Note that in the case of removed base, this would provide counter force along the equation axis to keep some of the acid in CO2 form.
- Renal compensation: increased H+ secretion, increased ammonia synthesis, increased bicarb reabsorption / production.
[edit] Anion gap
- Recall that metabolic acidosis can result from a depletion of HCO3.
- The chief purpose of calculating the anion gap is to determine the source of HCO3 depletion; that is, to determine the etiology of metabolic acidosis.
- Recall from general chemistry that charges in a solution like to be in equilibrium; the body maintains a certain equilibrium of positively charged ions (cations) to negatively charged ions (anions).
- In general, the anions and the cations are in equilibrium (as with all solutions).
- We can simplify this to a short equation if we only take into account the major ions:
- [Na+] = [Cl-] + [HCO3-] + [unmeasured anions]
- The anion gap is the difference in the cations and the anions.
- Note that we drop the unmeasured anions term because...well...they are unmeasured so we don't know the value.
- Unmeasured anions include lactate, ketones, proteins, phosphate, citrate, and sulfate to name a few.
- These will become causes of acidosis when they are aberrently elevated.
- Anion gap = [Na+] - [Cl-] - [HCO3-]
- Normal anion gap = 140 - 105 - 24 = 11 mEq / L
- This makes sense because Na is the highest concentration of cations, Cl is the major important anion, and HCO3- is the variable.
- Note that we drop the unmeasured anions term because...well...they are unmeasured so we don't know the value.
- So what does an increased anion gap mean?
- Recall that a normal anion gap = 140(Na+) - 105(Cl-) - 24(HCO3-) = 11 mEq / L.
- An increase in the anion gap means that there is less HCO3 to subtract from the Na or there is less Na from which to subtract the HCO3.
- Metabolic acidosis generates an increased anion gap because of a deficiency of HCO3 secondary to over production of metabolic acids like lactic acid, ketone body acids, or toxins.
- So what does a normal anion gap mean?
- It could mean that there is no acid-base problem. :)
- It could mean that the metabolic acidosis is caused by renal tubular acidosis, here's why:
- When HCO3- is lost (loss of a base leads to acidosis) at the renal tubule (perhaps because of diarrhea in which high volume requires lots of HCO3- secretion to maintain filtrate pH; perhaps because of poor HCO3 reabsorption at the PCT) it can be exchanged for Cl-.
Is this exchange an active exchange like Na for K in water balance or is it indirect like "well because HCO3 wasn't reabsorbed and yet the filtrate must be ionically balanced, Cl gets reabsorbed"?
- When HCO3- is exchanged for CL- at the renal tubule, the anion gap doesn't change, yet HCO3- is being depleted. That is, when metabolic acidosis occurs without an anion gap, one knows that the renal tubule is the source of HCO3 depletion.
- Metabolic acidosis generates a normal anion gap because of a deficiency of HCO3 secondary to over excretion of HCO3 at the renal tubule.
- Common causes of anion-gap acidosis:
- MULEPaKS
- Methanol
- Uremia (renal failure, urine in the blood)
- Lactic acid
- Ethylene glycol
- pAldehyde
- Ketone body acids
- Salicylates
[edit] Metabolic alkalosis
- Definition: "any abnormal function that results in a gain of base or loss of acid (especially the gain of bicarbonate but not the loss of H2CO2)".
- Recall that gain of bicarb or another base will push the HCO3 / CO2 reaction toward CO2.
- Recall that loss of acid will drag the HCO3 / CO2 reaction toward H+ / HCO3.
- Common causes:
- Acid loss: vomiting (stomach juices have lots of H+), hyperaldosteronism (excessive H+ loss at the kidney), hypokalemia (excessive H+ loss at the kidney via the H-K exchanger that will reabsorb K by sacrificing H+ to the filtrate)
- Base gain: excessive alkali intake
- Compensation:
- Chemical buffering: 1/3 in the cell compartment
- Pulmonary compensation: hypoventilation to increase PCO2 (but cannot fully compensate)
- Recall that HCO3- cannot be generated as quickly as acid when the HCO3 / CO2 equation is shifted toward H+ / HCO3.
- So increasing PCO2 will compensate for excessive acid loss by pushing the equation toward H+ / HCO3 and thus regenerating some of the acid.
- So increasing PCO2 will compensate for base gain by pushing the equation toward H+ / HCO3 and regenerating acid (faster than it regnerates HCO3) to counter the excess in base.
- Renal compensation: reabsorb less PCO3 and therefore lower the plasma HCO3.
- Note that this will compensate for increased base by decreasing another base: HCO3.
- Note that this will compensate for decreased acid by equalizing the ratio of acid to base.
[edit] Davenport diagram: A magic, visual explanation

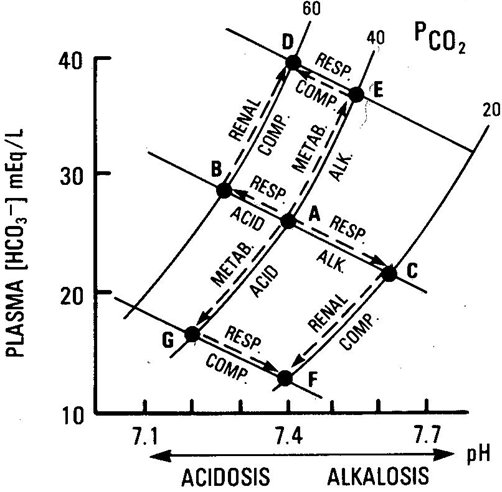

[edit] Examples of acid-base values
| pH | PCO2 (mmHg) | [HCO3-] (mEq / L) | State | |
|---|---|---|---|---|
| 7.08 | 49 | 14 | Respiratory acidosis + Metabolic acidosis (pulmonary disease -> low O -> high lactic acid) | |
| 7.32 | 28 | 14 | Metabolic acidosis | Respiratory compensation |
| 7.40 | 40 | 24 | Normal | NA |
| 7.51 | 49 | 38 | Metabolic alkalosis | Respiratory compensation |
| 7.53 | 20 | 16 | Respiratory alkalosis | Renal compensation |
| 7.62 | 20 | 20 | Respiratory alkalosis | Respiratory compensation |
- stopped here on 03/30/11.
